Anal cancer staging
Monique Maas and Doenja Lambregts
Radiology department of the Netherlands Cancer Institute Amsterdam
Anal cancer is a rare malignancy with a worldwide
incidence of approximately 1.5 per 100.000.
Almost all anal cancers are
squamous cell carcinomas.
Imaging plays a vital role in the staging and
treatment planning of anal cancer.
The diagnostic work-up consists of
proctoscopy with biopsies, MRI of the pelvis, ultrasound (with fine needle
aspiration) for the inguinal nodes, and CT or FDG-PET for the detection
of further nodal and distant metastasis.
Chemoradiation (CRT) is the
standard treatment for most anal cancers, after which 80-90% of patients
achieve a complete remission.
In patients with residual tumor, additional
surgery is required.
Some patients with small tumors at the perianal skin may
be managed with primary local excision instead of CRT.
Nodal involvement is
common in anal SCC and is usually treated with a radiation boost on the regional
lymph nodes.
Introduction
Checklist for staging
This is a checklist for the
structured reporting of anal cancer at baseline staging.
All these items will be discussed in the following chapters.
When reporting a restaging or follow-up MRI after chemoradiation, the report should include:
- Description of the degree of fibrotic transformation of the tumor bed.
- Estimation of the likelihood of the presence of vital residual or recurrent tumor within the fibrosis.
- Description of the size of any remaining lymph nodes and how they have changed in size compared to the previous imaging examination.
Anatomy
Anal cancer can be
subdivided into anal canal and anal margin (perianal skin) cancer.
Anal margin
cancers arise from the ±5 cm of perianal skin caudal from the anal margin.
They
are often more superficial and slow-growing tumors, that may be cured with
local excision or local radiation (if T1).
Tumors of the anal canal are often
more advanced (T2+ stage) and are treated with definitive chemoradiotherapy (CTR).
The dentate line marks the transition between columnar rectal mucosa and squamous anal
mucosa.
Tumors above the dentate line often spread to the mesorectal, internal
iliac and obturator lymph nodes, while tumors below this line typically spread to the
inguinal and external iliac nodes.
Staging anal cancer
T-stage
T-stage in anal cancer is primarily based on tumor size with the exception of T4 stage, which is invasion of adjacent organs:
- T1: tumor < 2 cm
- T2: tumor 2-5 cm
- T3: tumor > 5 cm
- T4: tumor of any size with adjacent organ invasion
In the Tis category Carcinoma in situ, Bowen disease, high-grade Squamous intraepithelial lesion (HSIL) and anal intraepithelial neoplasia II-III (AIN II-III) are included.
Choosing the right plane
Note that to determine the T-stage you should measure
the longest possible tumor diameter.
To do so, be sure to evaluate the tumor in
multiple planes and look for the longest tumor dimension.
Images
In this example, measuring
the tumor in the axial plane would falsely suggest a T1 tumor.
When measuring the
longest tumor axis in the coronal plane, the tumor stage is T2.
Tumor location
Describing the tumor location and whether the perianal skin, different layers of the anal sphincter and pelvic floor are involved does not impact the T-stage but is relevant to help guide radiation and/or surgical treatment planning.
- The internal sphincter (1) typically has an intermediate to low signal intensity on T2W MRI.
- The fatty intersphincteric plane (2) has a high signal .
- The striated muscle layers of the external sphincter (3), puborectalis (P) and levator ani (LA) have a low signal intensity.
When describing involvement of the internal and external anal sphincter, it is useful to describe both the cranioucaudal extent (e.g. upper half, lower half or full length of the anal canal) as well as the level of circumferential involvement (e.g. from … till … o’clock).
When you describe the location of the tumor, mention the following:
- If the anal margin (perianal skin) and/or anal canal are involved.
- Which layers of the anal sphincter and pelvic floor are involved
- The extent of craniocaudal extension
- If the tumor extends above the anorectal junction into the rectum.
Image
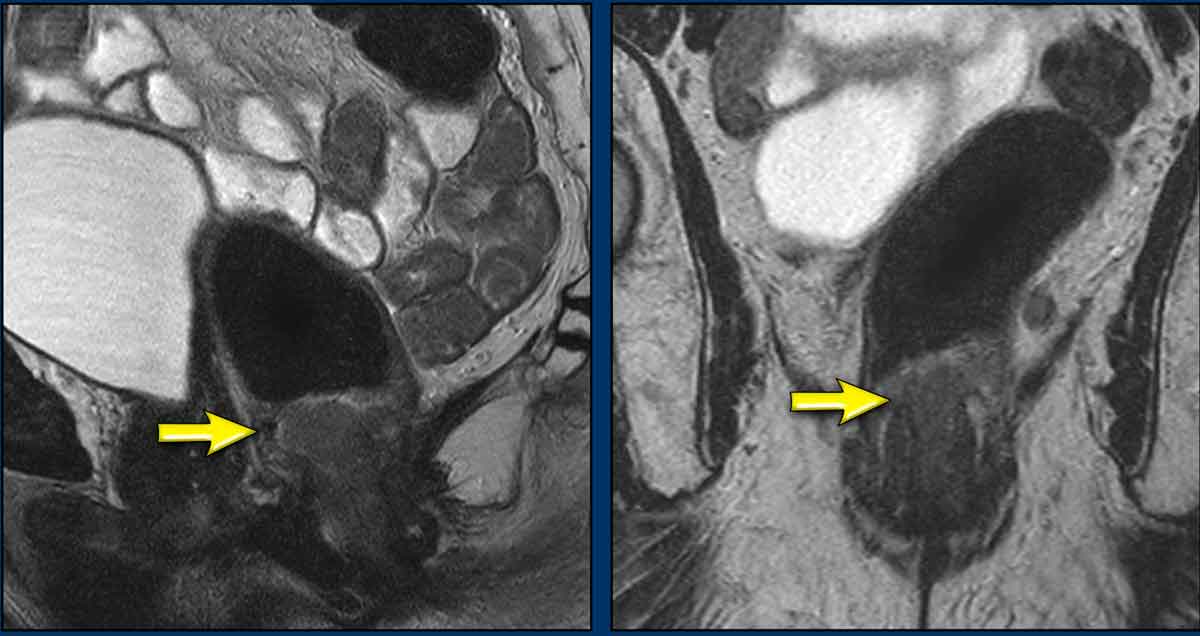
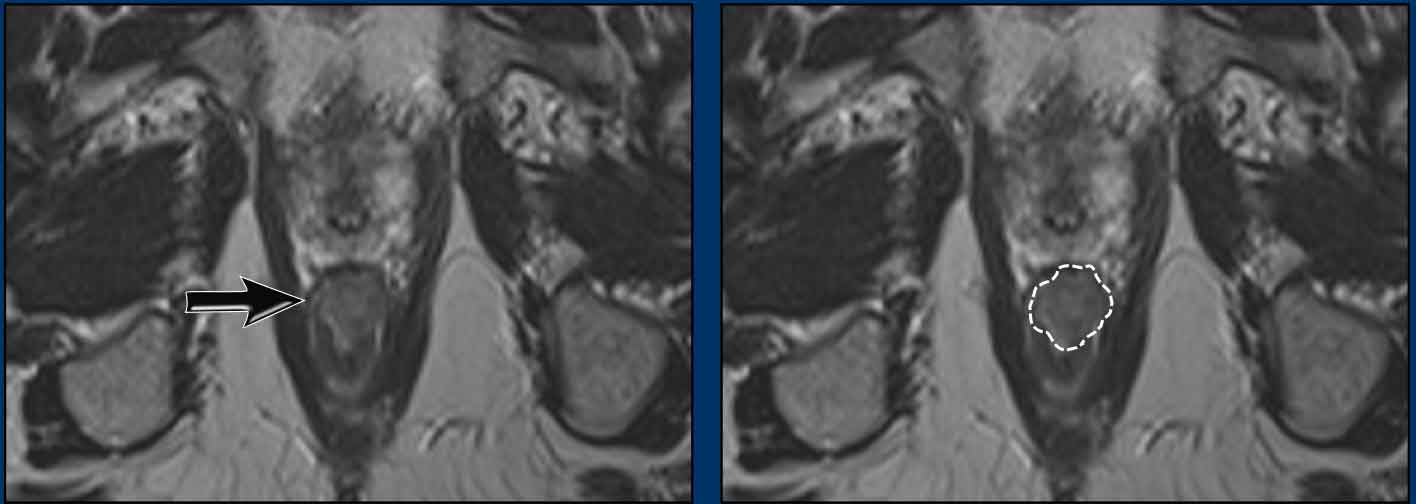
A tumor involves the distal 2/3 of the anal canal.
It invades the internal sphincter, intersphincteric space and external sphincter from ± 12 till 3 o’clock.
There is no involvement of the pelvic floor, rectum or anal margin.
Image
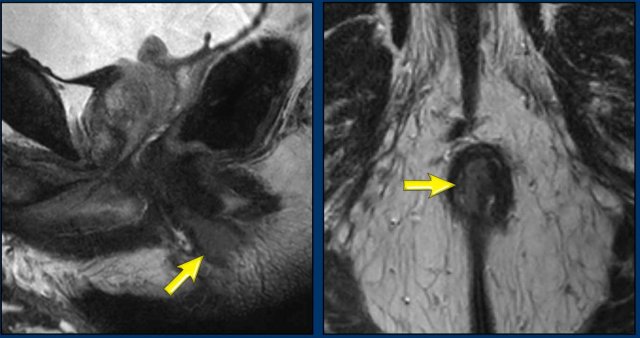
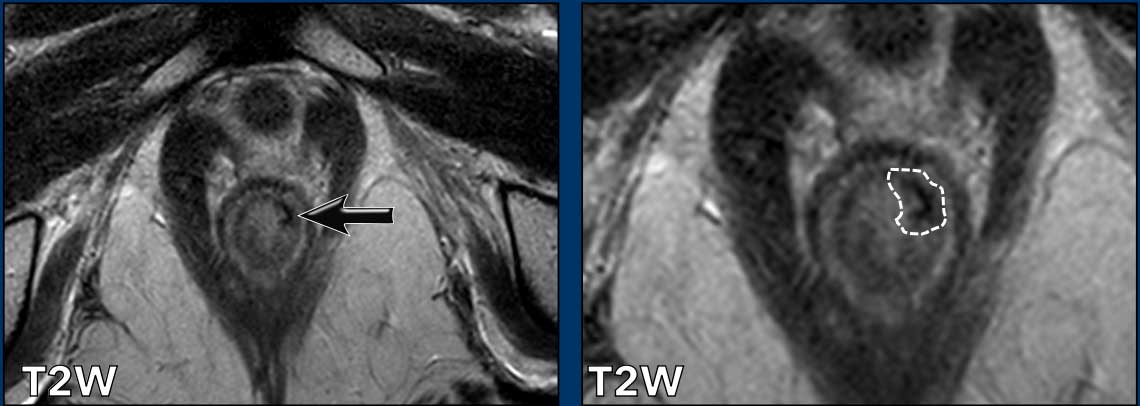
Another example showing a tumor that involves the proximal ½ of the anal canal.
It invades the internal sphincter, intershincteric space and external sphincter from 7-10 o’clock.
The tumor invades the puborectalis and levator ani on the right (arrow) and extends just above the level of the anorectal junction (dotted line) into the distal rectum.
N-stage
Nodal involvement occurs in about 25-45% of patients with anal cancer.
Unlike in rectal cancer where the N-stage is based on the number of suspicious nodes, N-staging in anal cancer is based on the location of N+ nodes:
- N1a nodes are nodes in inguinal, internal iliac, obturator and mesorectal regions (yellow)
- N1b nodes are the external iliac nodes (blue)
- N1c If there are both suspicious N1a and N1b nodes.
- M1 nodes are nodes along the common iliac vessels and aorta (purple). They are considered non-regional and typically entail incurable disease, that is managed with palliative chemotherapy.
Unlike in rectal cancer there are no widely accepted criteria to characterize anal cancer lymph nodes on MRI.
Some authors advise to adopt the criteria used for rectal cancer also for anal cancer staging.
Other reported criteria include:
- Short axis > 1 cm for mesorectal nodes
- Short axis > 1.5 cm for other nodes
- (Some advise >0.8 cm for parailiac nodes)
- Heterogeneity, necrosis, border irregularity
- Strong enhancement
These criteria do not have a strong evidence base and will inherently lead to both over- and understaging.
The most accurate nodal staging modality is 18F-FDG-PET-CT, with a sensitivity of 56-99% and specificity of 90-100% (1,2).
Ultrasound with fine needle aspiration is usually done as an adjunct to MRI and PET-CT, but in some centers it is used as the primary modality for loco-regional nodal staging.
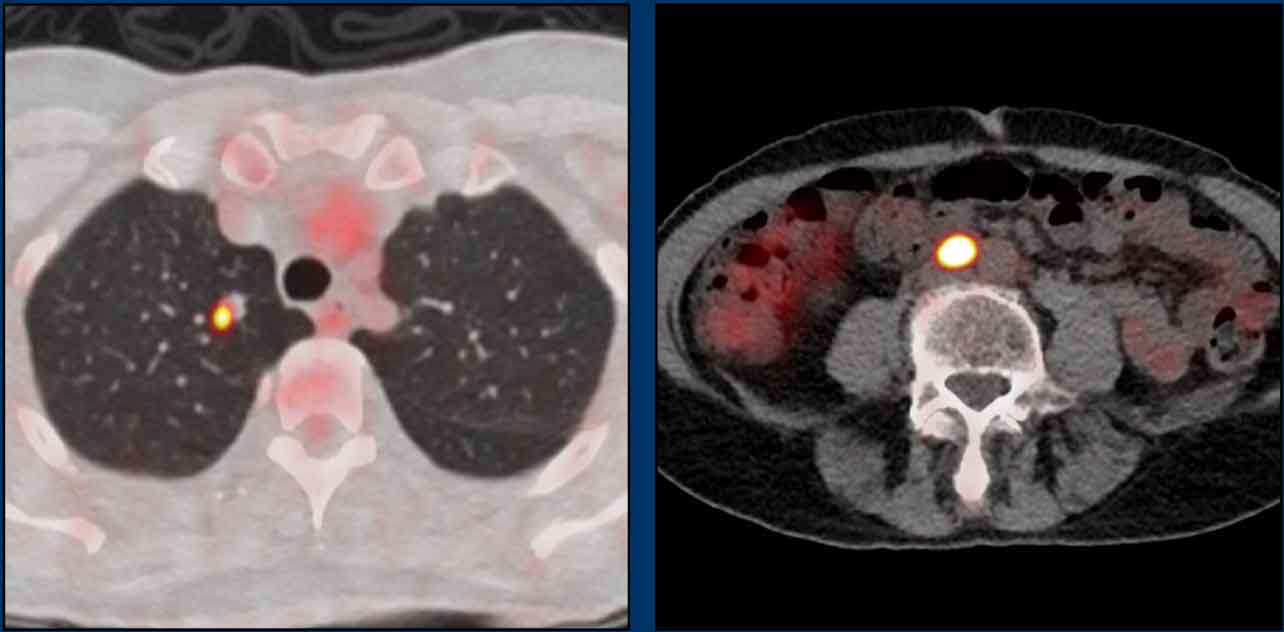
These images are of a patient with anal cancer.
Images
The MRI shows a clearly enlarged node (1.5 cm short axis diameter) adjacent to the internal iliac vessels, which was staged as N+ on MRI.
FDG-PET CT showed pathologic FDG uptake in the node, confirming it as N+.
In this case there are two small lymph nodes in the mesorectum that were considered as indistinct on MRI.
FDG-PET showed clearly increased FDG uptake in these small nodes, showing the added benefit of PET over MRI to stage anal cancer nodes.
The patient was finally staged as T2 N1a.
M-stage
Approximately 6% of patients with anal cancer present with distant metastases at diagnosis (3,4).
The prognosis is severely impaired by distant metastases with a 5-year median overall survival of only 10-20%.
The most common sites of distant metastasis are distant lymph nodes like common iliac nodes, para-aortic nodes and nodes above the diaphragm, followed by liver and lung metastases.
The recommended staging modality for M-staging in anal cancer is FDG-PET as almost all anal cancers are squamous cell cancers, which show clearly increased metabolic FDG uptake on PET.
Alternatively, a portal venous phase CT of the chest and abdomen may be performed.
Images
Two distant metastases that are clearly FDG-avid on PET: a suspicious nodule in the right lung and a distant para-aortic lymph node.
Restaging and follow-up after treatment
As mentioned before, when reporting a restaging or follow-up MRI after chemoradiation, the report should include:
- Description of the degree of fibrotic transformation of the tumor bed.
- Estimation of the likelihood of the presence of vital residual or recurrent tumor within the fibrosis.
- Description of the size of any remaining lymph nodes and how they have changed in size compared to the previous imaging examination.
The vast majority of anal cancers undergo definitive
chemoradiotherapy (CRT) which leads to a complete remission in ±80-90% of the
patients.
Maximum response rates are achieved after ±6 months at which time final
response evaluation with restaging should be performed.
The main goal of restaging is
to identify the ±10% of patients that still have vital residual tumor and
require additional surgical resection.
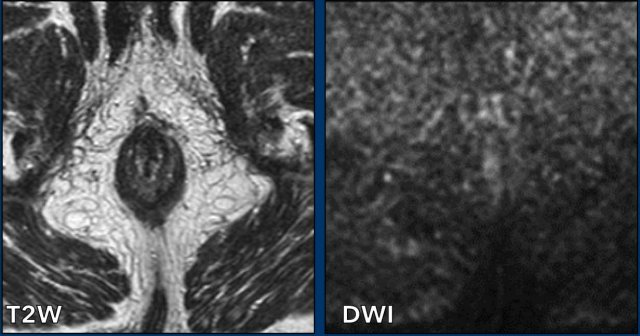
During restaging the diffusion-weighted
images are particularly helpful to detect residual tumor. Chemoradiation induces fibrosis with low signal on T2W images.
Absence of intermediate
to hyperintense residual signal on T2W images and absence of diffusion
restriction on DWI are signs that are highly predictive for a complete
response.
Images
Tumor (arrow) before treatment
with a suspicious mesorectal node.
Stage: cT2N1a.
Continue with the images after treatment...
Restaging
After treatment the
tumor has decreased in size.
There
is no residual intermediate signal mass on the sagittal and axial T2W images.
There
is only a small area of hypointense fibrosis
(arrows).
The
small dark spot on the ADC map represents ‘dark
through’ from
fibrosis (arrow).
This can be distinguished
from true diffusion-restriction, since there is no corresponding high signal on
the high b-value diffusion-weighted images.
Pitfall: timing of response
To assess the final response to treatment and decide whether or not to operate, imaging is best performed ±6 months after completion of chemoradiation.
If imaging is performed earlier, response may still be ongoing and presence of residual tumor is highly likely.
Some centers perform MRI at 6-10 weeks after chemoradiation.
These images should be regarded as an interim evaluation and baseline for
further follow-up.
Apart from MRI, clinicians will generally monitor response
by digital rectal examination (DRE) and clinical inspection.
When DRE is not feasible or if clinical
examination results in inconclusive findings, MRI can be used as an adjunct to
further assess the response.
Images
Anal tumor in the middle and lower third of the anal canal before treatment.
Continue with the follow up...
Evaluation after 6 weeks
The
first response evaluation was performed 6 weeks after the last
radiation fraction.
Images
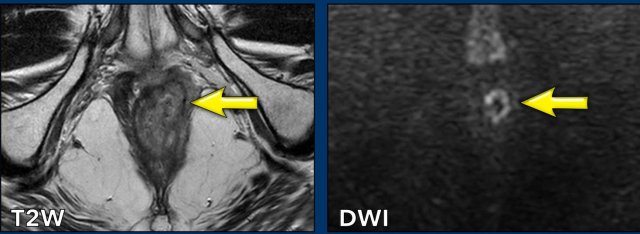
There is response, but residual tumor is still visible as intermediate
signal tissue on T2W MRI (black arrow) with corresponding diffusion restriction
(white arrow).
Continue with the follow up at 6 months...
Evaluation after 6 months
A second response
evaluation was performed at 6 months post-radiation.
Images
There is a complete response.
Local recurrence
Local recurrence is defined as biopsy confirmed reappearance of tumor or locoregional lymph nodes after an initial complete response, at least 6 months after the last radiation fraction.
Approximately 30% of patients treated with CRT will eventually have local failure (i.e. residual tumor after CRT or local recurrence during follow up).
About half of the recurrences occur in the first 2 years after completion of CRT.
Basaloid subtypes, higher stage tumors and HIV positive patients have a higher risk for local recurrence.
Basaloid carcinoma is a distinctive morphologic subtype of squamous cell carcinoma frequently associated with the human papilloma virus.
Local
recurrence is seen as a new intermediate signal mass on T2W with restricted diffusion on DWI or nodes showing growth during
follow up.
FDG-PET-CT can be used to confirm or rule out a local recurrence and simultaneously
look for distant metastasis.
Early detection of a local recurrence improves the
chance of successfull salvage surgery, which usually means abdominoperineal resection.
Images
This is an anal cancer before chemoradiation.
Continue with the images post treatment...
Images post CTR
There is a complete response with only a small thin area of fibrosis at the former tumor location at 1-3 ‘o clock in the internal sphincter (arrow).
There was no signs of diffusion restriction (scroll).
The rest of the internal sphincter shows some fuzzy intermediate to high signal, which represents radiation-induced edema (which shows no diffusion restriction).
Continue with the images after 2 years...
Images
There is a local recurrence 2 years after completion of CRT.
Note that the recurrence is larger than the primary tumor.
Extended abdomino-perineal resection after re-irradiation was required to salvage this recurrence.
Imaging protocol
The recommended MRI protocol mainly consists of high resolution T2W imaging in multiple planes with a slice thickness of ≤ 3 mm.
Diffusion-weighted imaging is mainly crucial in the restaging and follow-up setting because it increases the sensitivity of MRI to help detect areas of vital residual tumor within the fibrotically changed tumor bed.
MRI has a limited performance for N-staging and patients require additional FDG-PET/CT and/or ultrasound (with FNA) to more accurately assess the lymph nodes.
Sequence angulation
High resolution coronal T2W sequences are planned parallel to the anal canal to allow optimal visualization of the different layers of the anal canal.
Transverse sequences are planned perpendicular to the anal canal
Note that anal cancer may present with ‘skip lesions’
occurring at a distance from the primary tumour, i.e. higher up in the rectum
or mesorectal compartment.
Be sure to check for the presence of any
skip lesions on the large FOV images of the pelvis.
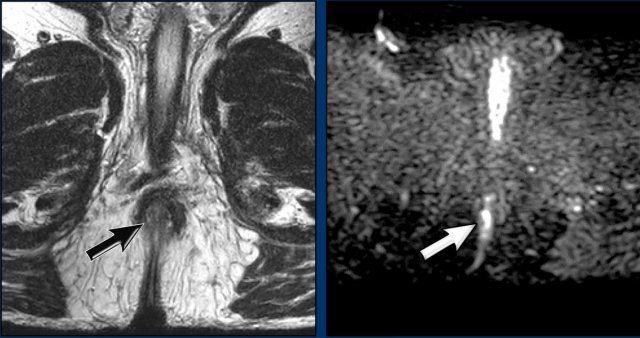
Image
There is a primary tumor located in the anal canal, presenting with a large skip
lesion in the mesorectum (arrow).
Anal cancer versus rectal cancer.
The table summarizes the main differences in staging and treatment between anal and rectal cancer.
Note that the definition of anal versus rectal cancer is based on the histology of the tumor and not its location.
Anal cancers are typically squamous cell carcinomas, while rectal cancers arise from large bowel mucosa and are typically adenocarcinomas.
Anal cancers may extend above the anorectal junction into the distal rectum or even be largely situated in the rectum.
Vice versa rectal cancers may extend into or be located for the majority within the anal canal.
When performing anal or rectal cancer staging, the radiologist thus needs to be informed about the underlying tumor histology to apply the correct TNM-staging.
The definitions for T4 disease are different for anal and rectal cancer.
Unlike in rectal cancer, invasion of the external sphincter (*) and pelvic floor muscles (*) is not T4 disease when staging anal cancer.
This example shows a tumor with a maximal diameter of 4.7 cm.
On the axial view the tumor involves the internal sphincter from 4-8 o’clock.
It extends into the intersphincteric plane and invades the levator ani on the right dorsal side (arrow).
In case of anal cancer this is staged as T2 (diameter 2-5 cm), while in case of a rectal cancer, the invasion of the external sphincter and levator ani would constitute a T4 stage.
Charity
All the profits of the Radiology Assistant go to Medical Action Myanmar which is run by Dr. Nini Tun and Dr. Frank Smithuis sr, who is a professor at Oxford university and happens to be the brother of Robin Smithuis.
Click on the image below to watch the video of Medical Action Myanmar and if you like the Radiology Assistant, please support Medical Action Myanmar with a small gift.