Neonatal Chest X-Ray
Joost van Schuppen, Laura Kox, Wes Onland, Robin Smithuis and Rick van Rijn
Radiology, Nuclear medicine and Neonatology department of the Amsterdam University Medical Centre
In this review we will discuss a systematic approach to the neonatal chest radiograph.
Close collaboration between neonatologists and radiologists is the key in achieving the correct diagnosis, since the radiographic features of many lung disorders overlap and the findings on the chest film can be rather subtle.
Clinical information like age of the neonate, gestational age and therapy with ventilation or surfactant is vital for the interpretation of the radiological findings.
We will first discuss a diagnostic approach based on radiographic and clinical findings and subsequently we will discuss the specific pulmonary diseases and complications.
Interpretations of tube positions, lines and catheter positions are discussed in Tubes and lines in neonates.
Introduction
Preterm infants show different types of pathology compared to
term infants.
For example, respiratory distress syndrome (RDS) is almost
exclusively seen in preterm infants.
Meconium aspiration (MA) on the other
hand, is seen in full term or late term neonates in combination with
meconium-stained amniotic fluid during labor.
Infants born between 34 and 37 weeks of gestation can have
diseases occurring in both preterm and full-term infants.
Invasive mechanical ventilation and surfactant therapy will have a huge impact on the radiographic findings and are essential clinical information for the radiologist.
Here is a list of common pulmonary disorders in neonates based on acute and chronic disease, complications of ventilation and congenital anomalies (Table).
* CPAM was previously referred to as congenital cystic adenomatoid malformation (CCAM) and presents as a mass of abnormal non-functional lung tissue
* TAPVR is total anomalous pulmonary venous return. The pulmonary veins do not connect to the left atrium, but enter the systemic venous circulation.
The approach to the neonatal chest film starts by looking at the technique of the radiograph.
Then the position of lines and tubes is analyzed.
See Lines and tubes in Neonates.
After these steps, the chest film can be interpreted for pathology.
This is done in a stepwise manner:
- Consider gestational age, postnatal age, and other clinical information, including respiratory support.
- Lung volume should interpreted, being either normal, hypo-inflated or hyper-inflated.
- In ventilated neonates positive end expiratory pressure (PEEP) should be noted on the request. (Normal PEEP 5-8 cm H2O)
- Interpretation of lung parenchyma, being either normal, lucent, or opacified.
- Pathology unilateral or bilateral.
The postnatal age of the neonate in days or hours is important as some disorders are acute conditions and some disorders at a present later stage. In addition, the course of illness and therapy so far should be taken into account.
Pattern Approach
Chest abnormalities can be divided into:
- Radiopaque
Other terms that are used to describe these abnormalities are high attenuation, or opacities. They can be fine or course linear, granular or reticulo-granular. They can be diffuse or focal. - Radiolucent
These are lucent abnormalities, being the result of hyperinflation of lung parenchyma, or abnormal collections of air. Depending on the location, these lucencies can follow pleura or mediastinal structures and are often sharply delineated.
Intraparenchymal abnormalities can be less circumscribed.
In some cases there is a combination of radiopaque and radiolucent abnormalities,
which makes the interpretation of the radiographic findings challenging.
For example, pulmonary interstitial emphysema (PIE)
can be regarded as lucencies, but can also be interpreted as radiating linear radiopaque abnormalities.
Notice that a diaphragmatic hernia can be a focal radiopaque or radiolucent
Radiopaque - High Attenuation
RDS
Respiratory distress syndrome in preterm neonates presents with low lungvolume, air bronchograms and symmetric fine granular opacities, ranging from mild disease to complete white out of the lungs.
TTN
Transient tachypnea of the newborn is seen in full term neonates with mild respiratory distress and presents with subtle increased lung volume, parahilar linear densities and pleural effusions.
MAS
Meconium aspiration is seen in full term and late term neonates and presents with coarse bilateral densities, that can be asymmetrical. There can be areas of atelectasis and air-trapping.
Atelectasis
Atelectasis can be seen in tube malpositioning and as complication, e.g. in RDS and meconium aspiration.
Radiolucent - Low attenuation
Air Leak
Most radiolucent lung abnormalities are the result of air leakage usually as a complication of positive pressure ventilatory support and present as:
- Pneumothorax
- Pneumomediastinum and pericardium
- Pulmonary Interstitial Emphysema (PIE)
- Pneumatocele
Hyperinflation
It can be the result of malpositioning of the tube with hyperinflation of one lung or in the case of atelectasis, there can be compensatory hyperinflation of other lung parts.
CPAM
CPAM
is the most common congenital lung malformation, but is still a rare disease.
There is a microcystic and a macrocystic form. The latter presents as a
radiolucent abnormality. Often in the first hours the lesion is not containing
air yet and can present as radiopaque.
Congenital diaphragmatic hernia
This is a birth defect of the diaphragm and can be a life threatening disease.
The herniation of abdominal organs into the chest results in underdevelopment of the lung.
Respiratory Distress Syndrome (RDS)
RDS is also known as hyaline membrane disease (HMD), idiopathic respiratory distress syndrome (IRDS) and surfactant deficiency disorder (SDD).
It is a result of deficiency of surfactant due to
immaturity of the lungs in preterm infants.
Surfactant production starts between 24 to 28 week of gestational age.
The incidence of RDS is inversely related to the gestational age at birth. It ranges from 50% in newborns at 26-28 weeks gestation and decreases to 25% in newborns at 30-31 weeks.
As a result of the surfactant deficiency, the alveoli will not contain air and cause diffuse bilateral micro-atelectasis, in combination with fibrin and cellular debris due to alveolar damage, which leads to poor compliance of the lungs and prevents the newborn to expand the lungs properly.
Endogenous production of surfactant will begin at approximately 36 - 72 hours regardless of the gestational age of the patient.
Therefore, the diagnosis of RDS is restricted to the first week of life.
Imaging
- Decreased lung volume on chest radiograph unless the patient is on ventilator.
- Bilateral symmetric ground glass opacities.
- Air bronchograms can be visible into the periphery.
- Poor definition of the cardiac silhouette and pulmonary vessels, as a result of less aerated lung parenchyma.
- Usually no pleural effusion.Radiological appearance can be asymmetric immediately after minimally invasive surfactant therapy (MIST) and can mimic for example neonatal pneumonia.
Imaging findings are often most
severe at birth, but may peak at 12- 24 hours after birth.
In many preterm neonates improvements
in treatment, including antenatal glucocorticoid administration, surfactant
replacement therapy and better ventilatory strategies have decreased the
prevalence of RDS.
When prolonged
mechanical ventilation is necessary, this increases the risk of lung injury and air
leak and can evolve into chronic lung disease.
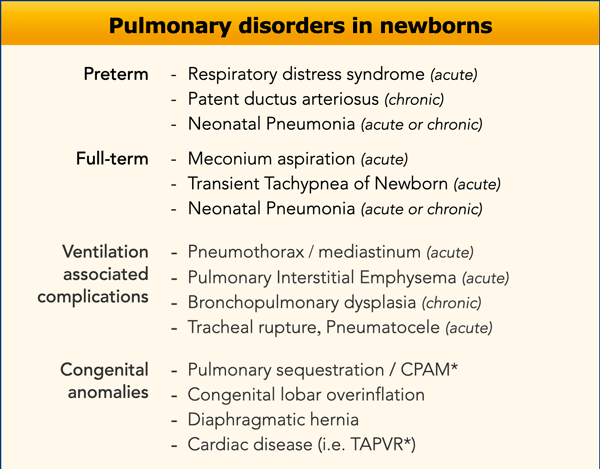
33 weeks + 5 weeks gestational age, day one.
First look at the image.
What are the findings?
Findings:
- Intubated patient.
- Good position of endotracheal tube (ETT). Hyperinflation.
- Symmetric granular opacifications with air bronchograms.
- Definition of heart and vessels is diminished.
This is a typical case of RDS.
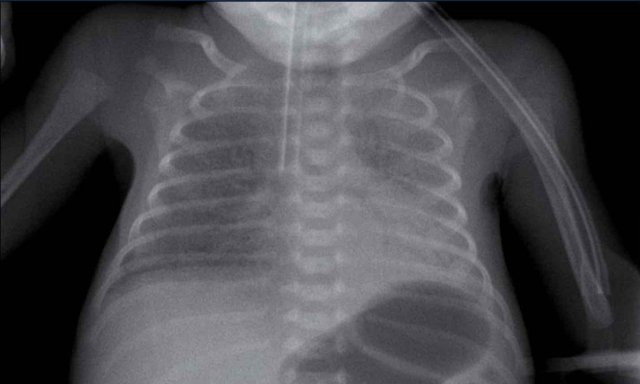
29 weeks + 1, day one. CPAP.
First look at the image.
What are the findings?
Findings:
- Reticulogranular opacification of lungs
- Air bronchogram
- Consolidation in the right lower lobe
- Heart, vessels and diaphragm are poorly defined.
- Malposition of umbilical vein catheter (arrow), probably in a pulmonary vein.
- Nasogastric tube (NG tube) in good position.
This is a severe case of RDS.
The differential diagnosis
includes pulmonary infection due to the asymmetric consolidation.
Grading
Grading of RDS can be performed but is not used when the patient is on invasive mechanical ventilation support.
There are 4 grades of staging RDS.
- Only mild ground glass or mild granular opacification of the lungs.
- Granular opacification with air bronchograms.
- Increasing consolidation of lung tissue leading to obscured borders of the heart and diaphragm.
- Complete pulmonary white-out.
As the lungs cannot expand
properly in RDS, hyperinflation in a preterm infant without mechanical
ventilation makes the diagnosis of RDS highly unlikely.
Image
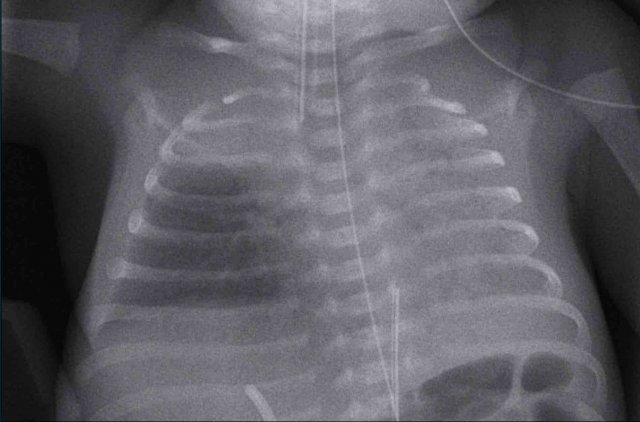
One day old neonate, 27 weeks of gestational age.
Granular opacification of both lungs.
Vessels and cardiac silhouette are well depicted.
Conclusion: RDS grade 1.
Peripherally inserted central catheter (PICC) line, curled in the right atrium. The PICC should be pulled back to the level of the superior vena cava and right atrium.
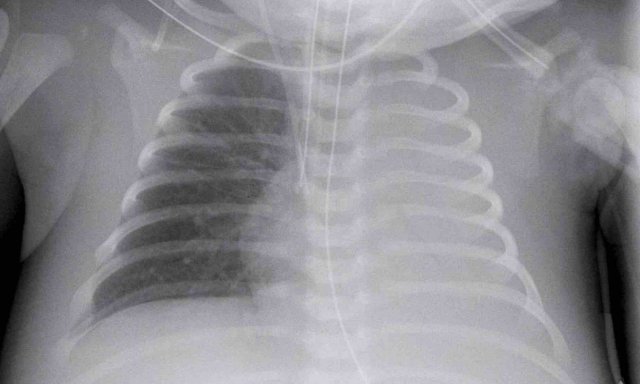
One day old neonate, 29 weeks of gestational age.
Image
- Hyperinflation due to CPAP.
- Granular opacification of both lungs with air bronchograms.
- NG tube in good position.
- Vessels and cardiac silhouette are harder to distinguish.
Conclusion: RDS grade 2.
One day old neonate, 26 weeks of gestational age.
Image
- Granular opacification of both lungs.
- Vessels and cardiac silhouette are hard to distinguish.
- Umbilical venous line properly positioned.
- Deep position of umbilical artery line, which should be pulled back to the level of T6.
- NG tube in situ.
Conclusion: RDS grade 3.
One week old neonate, born at 27 weeks of gestational age.
Image
- Deep position of the tracheal tube, which should be repositioned to the mid of the trachea.
- Diffuse granular opacification of both lungs.
- Air bronchograms.
- Pumonal vessels are not recognized anymore, but cardiac silhouette is still recognizable.
- Opacification of the left lower lobe caused by atelectasis.
No grading because this neonate is on mechanical ventilation.
Treatment
Preventive treatment of RDS consists of antenatal
corticosteroid administration in women at risk for preterm
delivery.
After birth, RDS may require treatment with exogenous
surfactant.
If the infant is supported with ventilation, surfactant is administered intra-tracheally
as a liquid bolus.
The clinical and radiological result of this treatment can often
be seen shortly after the administration of the surfactant.
The surfactant is often not evenly distributed, which can lead to more patchy aeration of the lung parenchyma.
Differentiation from other entities such as neonatal pneumonia
can be difficult.
Image
One day old boy, gestational age 25 weeks and 5 days.
- Grade 4 RDS with complete opacification of left lung and right upper lobe.
- After one bolus of surfactant the right lower lobe and the middle lobe are aerated.
- Good position of ETT and umbilical artery line.
- Malposition of the umbilical vein line in the portal vein. This should be removed.
Transient Tachypnea of the Newborn (TTN)
Transient Tachypnea of the Newborn (TTN) is also known as wet lung or retained fetal lung liquid.
TTN is a diffuse lung
disorder that results from delayed clearance of fetal lung fluid after birth,
leading to relative surfactant efficacy.
It is seen more frequently – but not
exclusively – in full term neonates after cesarean delivery.
Delayed clearance of fetal
lung fluid causes transient respiratory distress that improves within 48–72
hours after birth.
Imaging
- Mild increased lung volume.
- Interstitial edema resulting in perihilar linear densities.
- Subtle enlargement of the cardiac silhouette.
- Pleural effusions and fluid in the fissures.
- The radiological findings may be asymmetrical.
In many cases the clinical
presentation is mild and there is no need for a chest radiograph.
Only in some cases a chest
x-ray is performed to rule out complications.
The imaging findings may be
similar to those of RDS, showing diffuse granular opacities, or of pneumonia
with more coarse opacities.
Full term infant, 2 hours after elective caesarean section with some respiratory distress.
Image
- Mild hyperinflation
- Subtle interstitial linings on both sides
- Some pleural fluid on the right side (arrow).
- Skin fold on the right side
After
supportive therapy the respiratory distress disappeared the next day.
Image of a neonate with gestational age of 41 weeks with mild distress after birth.
No need for ventilatory
support.
Image
- Hyperinflation of both lungs
- Subtle interstitial markings
- Pleural fluid on the right side (arrow). This is very subtle.
- NG tube in situ
Clinical follow up was uneventful
41 Weeks neonate. 24 hours old.
Respiratory distress, no
ventilatory support
Image
- Marked hyperinflation of both lungs
- Increased vascular markings and interstitial markings
- Some interfissural fluid (arrow).
Spontaneous improvement within 48 hours.
Meconium Aspiration
Meconium aspiration results in diffuse pulmonary disease and it is the most common cause of significant morbidity and mortality among full-term and post-term neonates.
When intra-uterine hypoxia occurs, usually during labor, this
can lead to the fetus prenatally excreting meconium.
Inhalation of the meconium containing amniotic fluid results in a
chemical bronchiolitis with obstruction of the smaller airways
and surfactant dysfunction resulting in air trapping and
atelectasis.
Meconium aspiration can impede the transition from prenatal fetal
circulation to postnatal neonatal circulation.
In 10–15% of births meconium staining of the amniotic fluid is present, but only in 5% of these cases it will result in meconium aspiration.
The term Meconium Aspiration Syndrome is used to describe the
combination of sterile chemical pneumonitis and persistent fetal circulation or
persistent pulmonary hypertension of the newborn (PPHN).
Usually, this condition
presents within a few hours after birth.
Imaging
- Increased lung volume due to air trapping.
- Bronchiolitis can lead to atelectasis and air trapping.
- Complete obstruction of the bronchus can lead to atelectasis.
- Coarse, diffuse bilateral patchy or more linear opacification.
- Can present asymmetrical.
- Pleural effusions can be seen.
- In a later stage persistent pulmonary hypertension present as cardiomegaly on the x-ray.
Chest film of a full term newborn with meconium stained amniotic fluid.
Image
- Hyperinflation
- Course diffuse patchy consolidations on both sides.
- Some subtle pleural fluid on both sides (arrowheads).
- Good position ETT. Superficial position NG tube, still in esophagus.
- Deep position of umbilical vein line with tip in right atrium.
Chest film of a full term newborn with meconium stained amniotic fluid.
Image
- Good position of ETT, NG tube and umbilical vein line.
- Course patchy consolidations with relative sparing of the left apical lobe and right middle lobe.
- Mild hyperinflation.
Conclusion: chest radiograph in
keeping with meconium aspiration. Without staining of the amniotic fluid the
differential diagnosis would include neonatal pneumonia or TTN.
X-ray of a full term newborn with meconium stained amniotic fluid.
Image
- Hyperinflation.
- Asymmetrical patchy consolidation.
- Pleural fluid in the fissure on the right (arrow).
- Deep position of the tracheal tube.
- Malposition of the umbilical vein in right atrium and artery line in distal iliac artery.
Pulmonary hemorrhage
Pulmonary hemorrhage is uncommon in neonates.
Premature neonates are most at risk.
Other associated
factors are a persistent ductus arteriosus, surfactant treatment and mechanical
ventilation.
The exact etiology remains unclear.
The
radiographic signs are nonspecific and difficult to distinguish from other
disorders such as RDS.
This means that the diagnosis can only be made, when there is leakage of red blood or pink effusion from the
endotracheal tube.
Supportive measurements consist of ventilator support and sometimes xylometazoline or adrenalin, which is administered via the tracheal tube.
Mild hemorrhage can be hard to distinguish from RDS.
Massive bleedings show complete opacification of one or both lungs.
Image
- Premature infant, gestational age 33 weeks, with a history of RDS and blood via the endotracheal tube.
- Extensive consolidation of both lungs.
- Malposition of umbilical vein line. The line is positioned in the right atrium.
- Good position of ETT, and NG tube
The most likely diagnosis of this chest x-ray is RDS, but
given the clinical information of blood via the tracheal tube, the diagnosis is
pulmonary hemorrhage, possibly in combination with RDS.
Image of a neonate with gestational age of 41 weeks.
After 24 hours intubated for respiratory distress.
Blood via the endotracheal tube.
Image
- Good position of ETT, umbilical vein line, and NG tube
- Heterogeneous aeration of the lungs with hyperinflation of lower lobes.
- Diffuse interstitial
markings, more pronounced in right upper lobe and lower lobe.
The differential
diagnosis includes pneumonia and RDS in maternal diabetes.
In case of meconium
stained amniotic fluid, mecoinum aspiration could be considered as well, but this
is often more coarse.
Premature, 28 weeks of gestational age treated for RDS.
Blood via the tracheal tube.
Image
- Patchy opacifications in the right lung, due to hemorrhage.
- Atelectasis of the left lower lobe (arrow) with mild hyperinflation of the left upper lobe.
- Good position of ETT.
- Low position of umbilical vein line, NG tube in situ.
Persistent ductus arteriosus
The ductus arteriosus is
the connection between the pulmonary artery and the aorta.
Normally the ductus is open before birth and closes in term infants within
the first day after birth as arterioles feeding the wall of the ductus contract
in reaction to oxygen.
Preterm infants have fewer of these arterioles and sometimes an increase in oxygen does not lead to closure of the duct, which might result
in a high pulmonary blood flow.
The diagnosis usually is suspected 5 - 7 days after birth, when there is pulmonary overflow or systemic steal.
Prostaglandin inhibitors
Prostaglandin inhibitors can be used to close the open duct.
In some cases, surgical closure can be achieved either via a lateral
thoracotomy with a clip, or later in life via an intra-arterial approach using
a coil.
Prostaglandins
In neonates with a ductal-dependent cardiac disorder Prostaglandins can be used to keep the ductus arteriosus
patent, which can be life-saving.
Premature with previously normal chest radiographs.
Image
- Moderate enlargement of the heart in combination with ground glass changes of the lungs, in keeping with pulmonary edema.
- Deep position of the CVC in the right atrium, normal position of gastric tube.
Ultrasound confirmed a persistent ductus arteriosus.
Premature of 25 weeks and 5 days, now 2 month old. Treatement for RDS
- The first chest radiograph shows hyperinflation, and a
markedly enlarged heart with increased vascular markings and interstitial
markings.
NG tube and duodenal tube in situ. - After 5 days of treatment there is less hyperinflation and normalization of the heart size and vascular markings.
There still remain some interstitial markings, in keeping with BPD.
Bronchopulmonary Dysplasia
Bronchopulmonary dysplasia (BPD) also known as chronic lung
disease of the premature, is a disorder of lung injury and repair originally
ascribed to positive-pressure mechanical ventilation and oxygen toxicity.
BPD is nowadays a purely clinical diagnosis characterized by the requirement of oxygen for at least 28 days in an infant born at less than 30 weeks of gestation.
Before the advent of surfactant replacement therapy, chest
radiographs of infants with classic BPD demonstrated coarse reticular lung
opacities, cystic lucencies, and markedly disordered lung aeration that
reflected alternating regions of alveolar septal fibrosis and hyperinflated
normal lung.
Nowadays after the introduction of prenatal steroids and
postnatal surfactant and more sophisticated ventilatory support, BPD is
infrequently seen in infants with a birth weight of more than 1200 grams and
over 32 weeks of gestational age.
However, despite these advances in neonatal care, the
prevalence of BPD has changed little over the last decades due to the treatment
and improved survival rate of even more very preterm infants.
As a result of these changes, the international criteria for the diagnosis BPD were changed from 28 days postnatal age to 36 weeks postmenstrual age. Therefore, the term BPD should be avoided before this postmenstrual age.
Premature, born at 27 weeks of gestational age. Now 6 weeks of age.
History of intubation and mechanical airway support.
Image
Bilateral perihilar opacification and increased interstitial markings.
Given the history, BPD is the most likely diagnosis.
Premature, born at 27 weeks of gestational age. Now 8 weeks
of age.
History of extensive mechanical ventilation with prolonged
need for oxygen support.
Image
Bilateral perihilar opacifications with a coarse interstitial pattern as a sign of chronic small airway disease. NG tube in situ.
Given the history in combination with the radiological findings BPD is the most likely diagnosis.
Premature at 28 weeks, now 7 weeks of age, need for ventilatory support.
Image
Bilateral diffuse
interstitial markings, with some atelectasis on the left.
Given the history in combination with the radiological findings, BPD is the most likely diagnosis.
Atelectasis
Atelectasis often occurs due to malposition of tracheal
tubes, or to low positive pressure when using invasive mechanical ventilation.
Failure to clear mucus or secretion can cause atelectasis by
plugging of mucus.
Deficiency of surfactant can cause micro-atelectasis, leading
to diffuse atelectasis.
Treatment depends on the cause of the atelectasis, such as change of tracheal tube position, change in ventilatory support (pressure), alternating position from side to side, or in case of surfactant deficiency, intratracheal administration of surfactant.
Image
Complete atelectasis of the
left lung due to selective intubation on the right. PICC line in situ with tip
in superior vena cava. NG tube in situ.
This preterm neonate was treated for respiratory distress, probably due to pneumonia.
Image
Bilateral perihilar streaking can be seen, making the diagnosis of pneumonia most likely.
There is atelectasis
of the right middle lobe, probably due to mucus impaction of the bronchus.
Notice the position of the nasogastric catheter indicating mediastinal shift to
the right.
This is a neonate of 41 weeks gestational age, who was treated for asphyxia, including hypothermia treatment. Sudden respiratory distress.
Image
Bilateral opacification of the upper lobes most likely due to atelectasis.
There is some subtle streaky opacity most
pronounced retrocardial in the left lower lobe. This could be some atelectasis aswell.
Neonatal Pneumonia
Pneumonia can be difficult
to distinguish from other entities such as RDS or bronchopulmonary dysplasia.
In the majority of cases the clinical course, together with intratracheal sputum cultures and biochemical parameters are leading in the diagnosis of pneumonia.
Without signs of infection, a consolidation on a chest radiograph is unlikely to be caused by pneumonia.
Risk factors for neonatal pneumonia are:
- Preterm infants with ventilatory support and intubation.
- Full-term newborns after prolonged rupture of membranes or after maternal infection.
- Secondary after meconium aspiration.
- Transplacental (TORCH) infections
- Perinatal infection, such as E. coli or group B streptococcus, viral. Miscellaneous agents as Chlamydia trachomatis can be found.
Neonate, GA 37+6 weeks, respiratory distress, treated with CPAP after delivery. History of maternal infection.
First study the image.
What are the findings.
Image
Bilateral increased lung volume with asymmetric increased opacification of the lungs with subtle consolidation of right upper lobe (black arrow) and left lower lobe (white arrow).
Hyperinflation of left upper lobe.
This child developed signs of infection, both clinically and in the laboratory findings.
The radiographic findings were attributed to neonatal pneumonia.
A full term neonate, with respiratory distress after 24 hours.
Image
Hyperinflation of both lungs and cardiac enlargement with increased
interstitial markings and vascular markings. No pleural fluid.
The differential diagnosis includes TTN and neonatal pneumonia. After 48 hours there was no improvement of the respiratory distress, and the neonate developed signs of infection.
One
might argue that there could be a combination of TTN and pneumonia.
Ventilation associated complications
Air leakage as a result of barotrauma in newborns present as:
- Pneumothorax
- Pneumomediastinum and pericardium
- Pulmonary Interstitial Emphysema (PIE)
- Tracheal rupture
- Pneumatocele
Pneumothorax can occur spontaneously or as a complication of
positive pressure ventilatory support.
The introduction of surfactant treatment and improved
ventilatory support has significantly decreased the incidence.
Rupture of terminal airways results from high pressure
insufflation of collapsed lung tissue. This causes air leakage into the
pulmonary interstitium, lymphatic system or pleural space.
A specific finding on neonatal chest films is air leak
tracking along the bronchi termed pulmonary interstitial emphysema (PIE).
Air within the interstitium can cause stiff and incompliant
lungs and diminished blood flow, leading to a reduced blood oxygenation.
Pneumothorax can cause collapse of a lung, but in newborns the lungs are
relatively non-compliant and volume loss is often limited.
When additional pneumomediastinum is present, a ‘lifted thymus’ can be seen, also known as the ‘Spinnaker Sign’.
Pneumothorax
Neonate 3 days old with RDS. Gestational age: 34 weeks.
First study the image.
What are the findings?
Image
- Increased lucency of the right pleural space.
- Increased density of the partially collapsed right lung.
- Mediastinal shift to the left as a result of the pneumothorax under tension.
- Pneumomediastinum causing ‘lifting’ of the thymus.
Pitfall
In newborns an important pitfall is the presence of a skin fold, which can be mistaken for a pneumothorax.
Skinfolds do no follow anatomical borders and can be traced outside the chest cavity or cross-over pulmonary vessels.
Image
A term newborn with an abdominal sepsis.
There are signs of
fluid overload with accentuated blurry vessels. Skinfolds project over the right lower lung (arrows).
The lines cross anatomical borders, e.g. diaphragm and do
not follow pleura or lungs.
Left arm projecting over hemi-thorax, resulting in sharp radiopaque line (arrowhead).
Images of a neonate with respiratory distress.
37 weeks gestational age
After primary caesarean section.
Left image
Hyperinflation on the left side.
Mild displacement of the midline
structures to the right.
A pneumothorax is visible on the left side (arrow)
Right image
On the follow up chest x-ray, the pneumothorax has spontaneously resolved.
A pneumothorax can be very subtle, since in many neonatal
pulmonary disorders the lungs are not compliant and will not collapse.
Often in
a supine neonate, the pneumothorax only manifests ventrally.
Sometimes only sharpening of the mediastinal structures with
unilateral lucency is noted.
Image
Full term infant shortly after birth with mild pulmonary
distress.
- Mild diffuse linear opacification of both lungs in keeping with TTN.
- Bilateral pneumothorax (arrowheads).
- Sharpening of the mediastinal structures on both sides as a result of interface between mediastinum and pneumothorax.
Due to fluid in the lung parenchyma these lungs were stiff and did not collapse.
Neonate 32 weeks gestational age. Treated for RDS via CPAP, with sudden respiratory distress.
First study the image.
What are the findings?
Image
There is a a pneumothorax on the right side.
Midline structures are displaced to the left.
The left lungs
shows reticulonodular markings in keeping with RDS.
The right lung is not completely collapsed due to stiffness of the parenchyma in RDS and fluid.
Pneumomediastinum
Pneumomediastinum is recognized as air inside the
mediastinum.
The classical sign is the so-called spinnaker sign
(arrowhead).
This is caused by the thymus being ‘lifted up’ from the lower
mediastinum by the mediastinal air.
First study the image.
What are the findings?
Image
- A residual ventral pneumothorax can be recognized by the sharp paracardial and mediastinal contour on the left side.
- On the right
side there is a drain in situ, with no apparent residual pneumothorax, but subcutaneous emphysema, due to air leak with drain.
- Pneumomediastinum is recognized as air inside the mediastinum, where there is air between the thymus and heart figure (arrowhead).
- Skinfold over right hemi-thorax.
Image
Air can be recognized
between the thymus and the heart, which indicates a pneumomediastinum.
No apparent pneumothorax is
recognized.
The thymus
is uplifted on both sides (arrows).
A cross table lateral view can help
to confirm the presence of a pneumothorax or a pneumomediastinum.
Image
Air can be recognized between the
thymus and the heart, which indicates a pneumomediastinum.
No apparent pneumothorax is recognized.
A full term neonate after meconium aspiration.
Pneumomediastium, in combination with subcutaneous emphysema. NG tube in situ.
Pulmonary Interstitial Emphysema (PIE)
Pulmonary interstitial emphysema (PIE) is leakage of air into
the perivascular and peribronchial spaces as a result of rupture at the
bronchiolo-alveolar junctions.
PIE is recognized as either small bubbles or linear air
collections along the bronchovascular bundle radiating from hilum to the
periphery.
PIE can be bilateral or unilateral.
Once PIE is established, air may dissect centrifugally along
bronchovascular sheaths or lymphatic channels to form subpleural blebs, which
may rupture into the pleural space and produce a pneumothorax.
Image
Preterm infant born at 27 weeks gestational age.
Now 6 weeks old.
Mechanical ventilation for RDS.
First study the image.
What are the findings?
Findings:
- Bubbly air configurations bilateral indicating PIE.
- Note the small
mediastinal silhouette, caused by shrinking of the thymus as a result of
stress.
- Deep position umbilical vein line, tip likely in left atrium or pulmonary vein.
- Good position ETT. NG tube in situ.
Neonate, gestational age 30 weeks, day 1, sudden deterioration after MIST.
First study the image.
What are the findings?
Image
Bilateral radiating bubbly lucencies, with
hyperinflation of both lungs.
These are typical findings in PIE.
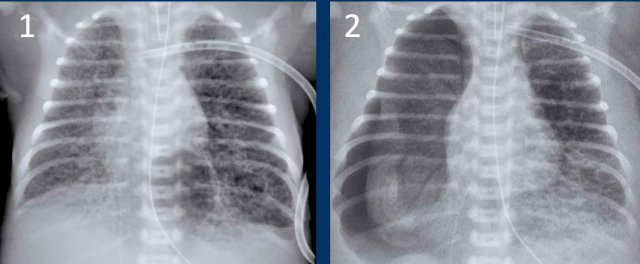
Neonate, gestational age 27 weeks, treated for RDS.
At age of 2 days sudden deterioration.
First study the images.
What are the findings?
Image 1
Bilateral reticulonodulair opacities in keeping with IRDS,
treated via CPAP.
Image 2
At the age of 2 days the X-ray
shows radiating lucencies in the left lung as a result of PIE.
These images are of a neonate gestational age 32 weeks, who was treated for RDS.
Several complications developed, including a pneumothorax on the left side, which was drained.
After
drainage there was a deterioration.
Image 1
The radiograph shows bilateral radiating bubbly lucencies due to bilateral PIE.
This is more pronounced on the left side.
Image 2
In follow up the child also developed a pneumothorax on the right side.
Congenital anomalies
The most common congenital anomalies in neonates are:
- Congenital pulmonary airway malformation (CPAM)
- Pulmonary sequester
- Congenital lobar emphysema
- Diafragmatic herniation
Congenital pulmonary airway malformation
Congenital pulmonary airway malformation (CPAM) was until recently know as congenital cystic adenomatoid malformation (CCAM).
It is a spectrum of bronchopulmonary foregut malformations.
There are three histological types:
- Type I (75% of cases) is composed of variable sized cysts, with at least one dominant cyst larger than 2 cm in diameter.
- Type 2 (10-15% of cases) is composed of smaller multiple cysts of the same size, less than 1 cm in diameter.
- Type 3 presents as a solid mass composed of micro cysts.
Image
1 week old child, born at 41 weeks gestational age.
On prenatal routine imaging, a cystic lesion was seen in the upper left
lobe.
The radiograph shows a delineated lucent area in the apical part of the
left upper lobe (arrow).
There is a slight mediastinal shift to the right.
Radiographic findings in CPAM
- In type I and II, multiple thin walled cysts containing air and varying in size are seen.
- In type III a solid lesion is seen.
- Infection of the cysts can cause air fluid levels.
- Large lesions cause mass effect with mediastinal shift and atelectasis of adjacent lung parenchyma.
- CPAM usually involves one lobe.
Contrast
enhanced CT scan is essential in the analysis of CPAM and sequesters.
Given that CPAM and sequester often are hybrid lesions,
feeding arterial vessels need to be visualized or ruled out before surgical
intervention.
Image
CT scan of the same patient as above.
The lucent lesion in the left upper lobe has a multicystic aspect.
Because the largest cyst has a diameter of more than 2 cm, this is classified as a
type I CPAM.
Images of a neonate, 40 weeks gestational age.
Antenatal suspicion of large CPAM on the left side.
At birth respiratory distress
Images
Radiograph shows a large round opacified lesion.
Severe displacement of
midline structures with atelectasis of the right lung.
Deep position ETT. NG tube in situ.
Because of the need of direct intervention a CT after IV contrast was
performed.
CT shows a large cystic lesion in the upper lobe of
the left lung, with displacement of vascular and bronchial structures.
The lesion has relation to any systemic vessel, Which excludes a sequester.
This is a CPAM, which is not yet aerated.
Pulmonary sequestration
A pulmonary sequestration is a segment of dysplastic lung
tissue, which is separate from the rest of the lung. It receives an anomalous
systemic vascular supply.
The most common type is the intralobar type, which is
situated within a normal lobe and has no own visceral pleura.
Usually there is
a normal venous return via the pulmonary veins.
In contrast the extralobar type is situated outside of the
normal lung and has a separate visceral pleural and a systemic venous return.
Most sequestrations are detected on antenatal ultrasound and present as a mass in the basal lobes, usually paramedian on the left.
Image
Neonate, 39 weeks gestational age, antenatal suspicion of CPAM left lower
lobe.
Radiograph shows a subtle, not well circumscribed lesion on the
left lower lobe.
CT at age of one month shows a mixed lesion, both cystic and
solid, with a large feeding artery from the descending aorta, in keeping with
sequester.
Imaging
- On radiographs a sequestration is often not detected.
- Prenatal
ultrasound often raises the suspicion of a sequestration, which is seen as a hyperechoic mass.
Management is controversial. Some advocate surgical resection because of the risk of infection
and cardiac failure due to the left to right shunting.
Others advocate a wait and
see approach, as these sequestrations can resolve spontaneously.
Before surgical management MR or CT is needed to analyze the blood supply and the type of the sequestration.
Congenital Lobar Emphysema
Congenital lobar emphysema, now known as congenital lobar
overinflation, is a condition in which there is hyperexpansion of a lobe of the
lungs.
Narrowing or weakness of a lobar bronchus causing a
check-valve mechanism is the most likely cause.
Symptoms depend on the degree of lobar hyperexpansion.
A mildly hyperexpanded lobe may be asymptomatic and decrease
in size with time and close follow-up is warranted in these cases. Progressive
hyperexpansion of a lobe can occur and cause significant, sometimes life-threatening,
symptoms. Those cases are treated with lobectomy.
Usually congenital lobar emphysema is detected on prenatal ultrasound.
Images of a term neonate with mild respiratory distress.
Antenatal there was suspicion
of a CPAM.
The chest radiograph and CT show hyperinflation of the right upper lobe with architectural distortion.
The right lower lobe is compressed, but probably has a normal architecture.
Displacement of
mediastinum and heart and atelectasis of the left lung.
Images of a 6 months old neonate with mild respiratory symptoms.
Radiograph reveals hyperinflation of left upper lobe with displacement of midline structures and some atelectasis of the lower lobe.
The CT confirms overinflation of the right upper lobe.
Diaphragmatic hernia
There are two types of hernia, the Bochdalek and the Morgagni type.
The diagnosis is often made prenatally.
The
Bochdalek hernia is seen in about 95% of cases and located on the
posterior side of the chest. Most often it is left sided (90%).
The
Morgagni hernia, also known as retrosternal or parasternal hernia, is located
parasternally, where the foramina of Morgagni are situated.
The severity of the disease is based on the degree of hypoplasia of the lung.
Imaging findings
- Total or partial opacification of one side of the thorax, with no or little aeration of the ipsilateral lung.
- Shift of the mediastinum to contralateral side.
- The finding of part of the digestive tract in the chest cavity is pathognomic for the diagnosis.
- Direct postpartum the digestive tract does not contain air, so it may go unnoticed or simulate an abnormality within the lungs.
Image
Full term neonate with shortness of breath.
Partial opacification of the left upper hemithorax.
Bowel structures are seen in the left hemithorax (arrow).
Shift of midline structures seen as deviation of the nasogastric tube (arrowhead)
Ultrasound demonstrated peristalsis in air and fluid containing structures confirming a large diafragmatic hernia .
These images are of a full-term neonate with shortness of breath.
Images
Partial opacification of
the left upper hemithorax.
Bowel structures are seen
in the left hemithorax.
Shift of midline structures seen as deviation of the nasogastric tube
(arrowhead).
The lateral chest film also demonstrates bowel loops in the chest.
Charity
All the profits of the Radiology Assistant go to Medical Action Myanmar which is run by Dr. Nini Tun and Dr. Frank Smithuis sr, who is a professor at Oxford university and happens to be the brother of Robin Smithuis.
Click here to watch the video of Medical Action Myanmar and if you like the Radiology Assistant, please support Medical Action Myanmar with a small gift