Ovarian cystic lesions
Wouter Veldhuis, Robin Smithuis, Oguz Akin and Hedvig Hricak
Department of Radiology of the University Medical Center of Utrecht, of the Rijnland hospital in Leiderdorp, the Netherlands and the Department of Radiology, Memorial Sloan-Kettering Cancer Center, New York, USA
Publicationdate
In this review the imaging features of normal ovaries and the most common ovarian cystic masses are presented.
In Ovarian Cystic Masses Part I a roadmap for the diagnostic workup and management of ovarian cystic masses is presented based on the findings of ultrasound and MRI.
Images can be enlarged by clicking on them.
On the iPhone application this results in hi-res images at full retina resolution.
Normal ovaries
premenopausal
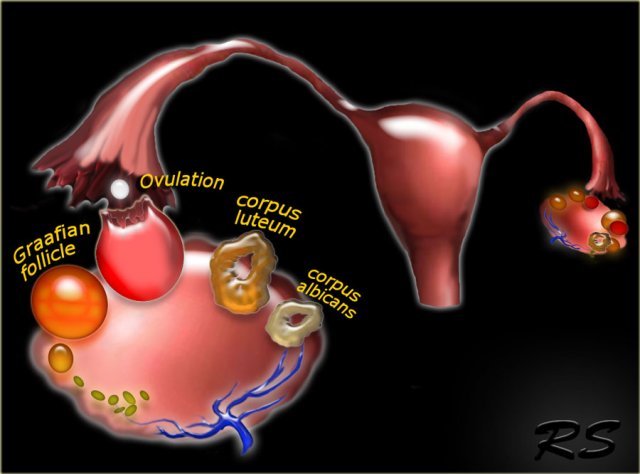
The normal ovary contains over two million primary oocytes at birth, about 10 of which mature each menstrual cycle.
Of the 10 Graafian follicles that begin to mature, only one becomes the dominant follicle and grows to a size of 18-20 mm by mid-cycle, when it ruptures to release the oocyte.
The other nine follicles become atretic and fibrous.
After release of the oocyte, the dominant follicle collapses, and the granulosa cells in the inner lining proliferate and swell to form the corpus luteum of menstruation.
Over the course of 14 days the corpus luteum degenerates, leaving the small scarred corpus albicans.
Graafian follicles
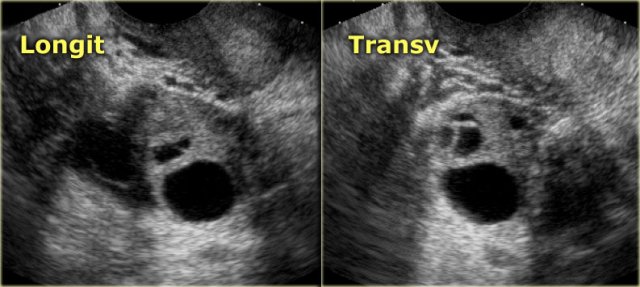
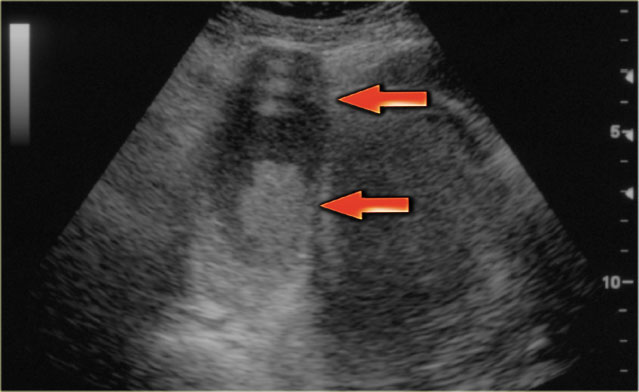
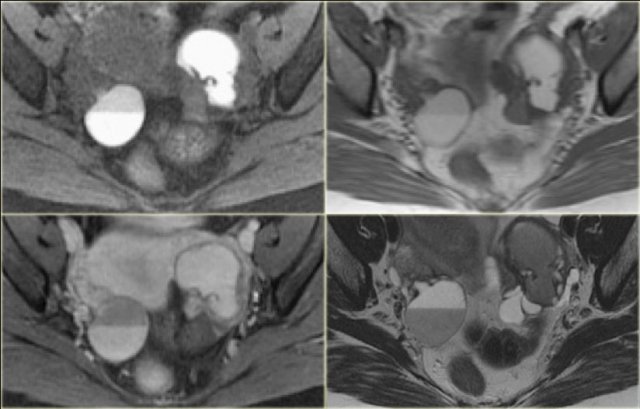
The normal ovary in pre-menopausal women contains small cysts.
The images show two normal ovaries with several anechoic, simple cysts consistent with Graafian follicles.
On T2-weighted MR-images the Graafian follicles are seen as bright cysts surrounded by darker solid ovarian stroma.
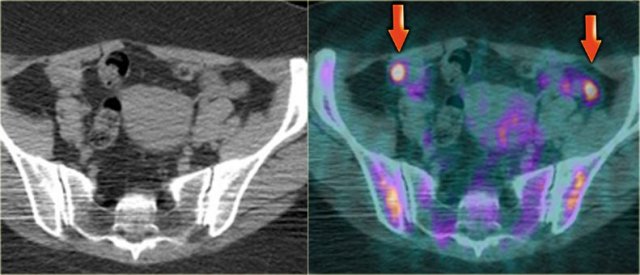
FDG-PET pitfall - normal premenopausal ovaries
In some pre-menopausal women the normal ovaries may be avidly PET positive, depending on the date in the menstrual cycle.
Because in pre-menopausal women a PET-positive ovary may be either an adnexal neoplasm or completely normal, it is important to be aware of the possibility of physiologic mid-cycle FDG uptake and to correlate this finding with the clinical history.
FDG-PET in pre-menopausal women should therefore preferably be scheduled in the first week of the menstrual cycle.
In post-menopausal women, the normal ovaries show only minimal uptake of FDG.
Any increased ovarian FDG uptake in this age group is suspicious for a possible neoplasm.
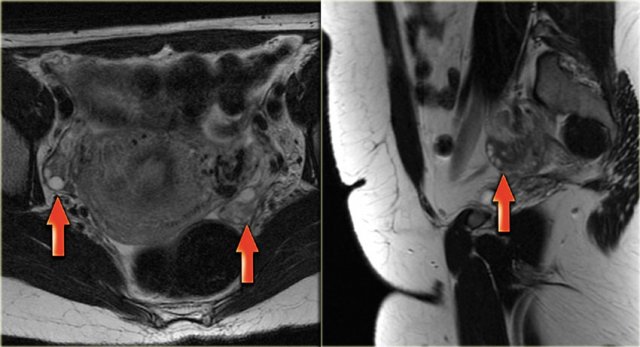
 LEFT: Postmenopausal woman. The ovary is a T2 dark tissue clump near the proximal end of the round ligament.RIGHT: T2 dark left ovary devoid of follicles. It is a bit prominent, but is still likely to be normal
LEFT: Postmenopausal woman. The ovary is a T2 dark tissue clump near the proximal end of the round ligament.RIGHT: T2 dark left ovary devoid of follicles. It is a bit prominent, but is still likely to be normal
Post-menopausal
Post-menopause is defined as 1 year or more of amenorrhea. In Western countries the average age of menopause is 51-53 years.
In post-menopausal women the ovaries are generally smaller and gradually stop forming Graafian follicles.
Note, however, that follicular cysts may persist several years after menopause.
In the coronal T2-weighted image of a postmenopausal woman the ovary is no more than a dark tissue clump near the proximal end of the round ligament.
The axial T2-weighted image also shows a dark left ovary, devoid of follicles.
Although a bit prominent, this is likely to be completely normal.
Only if, by chance, there happened to be prior imaging showing that the lesion was growing, your differential diagnosis would start with a benign solid lesion such as ovarian fibroma or fibrothecoma.
Functional cysts
By far the most common cystic ovarian lesions are benign functional ovarian cysts.
Functional cysts are Graafian follicles or corpora lutea that have grown too large or have bled, but are otherwise benign.
In the early post-menopause phase, 1-5 years after the final menstrual period, sporadic ovulatory cycles still may occur and ovarian cysts may be seen.
Even in late menopause, which is defined as more than 5 years since the final menstrual period, when ovulation is unlikely to occur, small simple cysts may be seen in up to 20% of women.
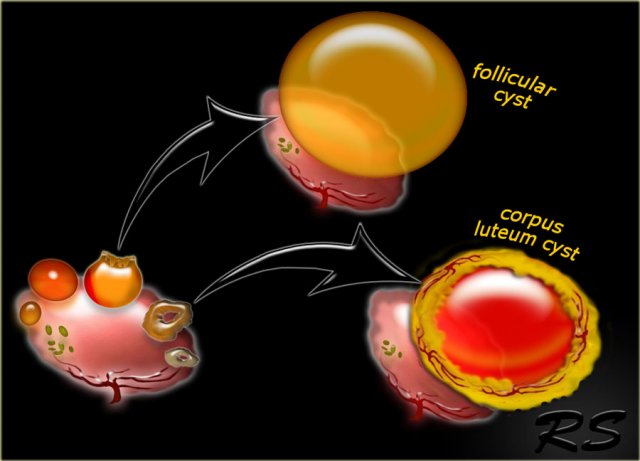
Follicular cyst
A dominant Graafian follicle sometimes fails to ovulate and does not involute. When it becomes larger than 3 cm, it is called a follicular cyst.
Follicular cysts are usually 3-8 cm, but may become much larger.
On ultrasound follicular cysts present as simple unilocular, anechoic cysts with a thin, smooth wall.
There should be no enhancing nodules or other solid components, no enhancing septations, and no more than physiologic ascites.
Follicular cysts will usually resolve spontaneously on follow-up.
Corpus luteum cyst
A corpus luteum may seal and fill with fluid or blood, forming a corpus luteum cyst.
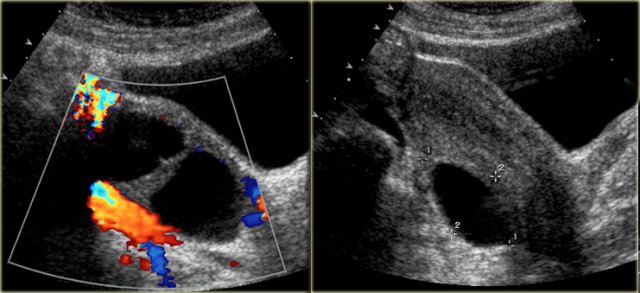
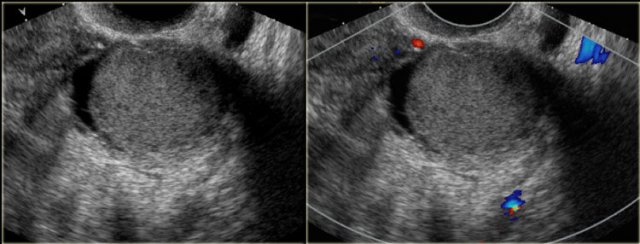
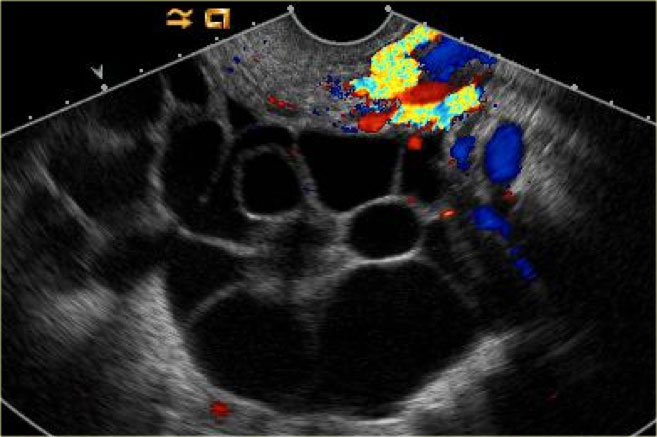
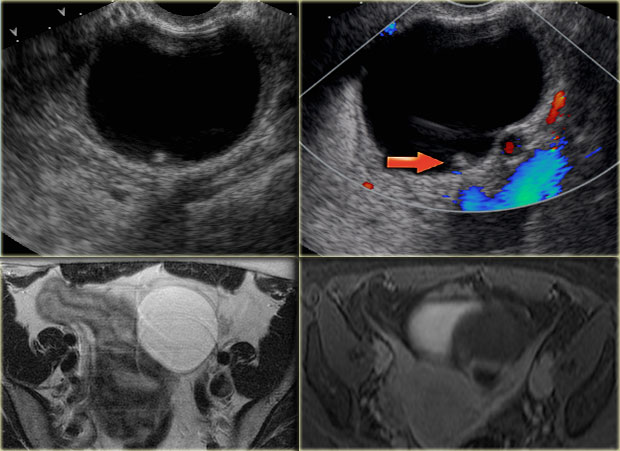
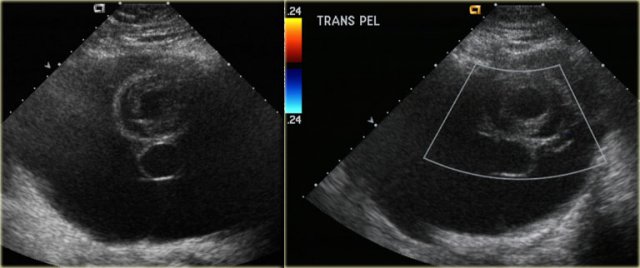
The transvaginal ultrasound images show a small complex ovarian cyst with wall vascularity on power Doppler analysis.
The characteristic circular Doppler appearance is called the 'ring of fire'.
Note, there is good through-transmission and no internal vascularity, consistent with a, partially involuted, corpus luteum cyst.
Remember that women who are on birth control pills usually won't form a corpus luteum, as birth control pills prevent ovulation.
On the other hand, use of fertility drugs that induce ovulation, increases the chance of developing corpus luteum cysts.
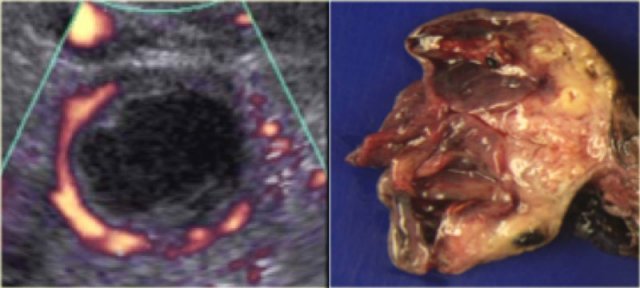
Another case with the typical the 'ring of fire' on ultrasound.
At pathologic examination the collapsed bloody cyst can be clearly seen.
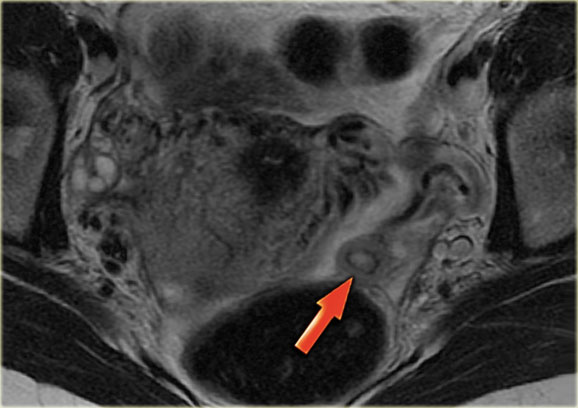
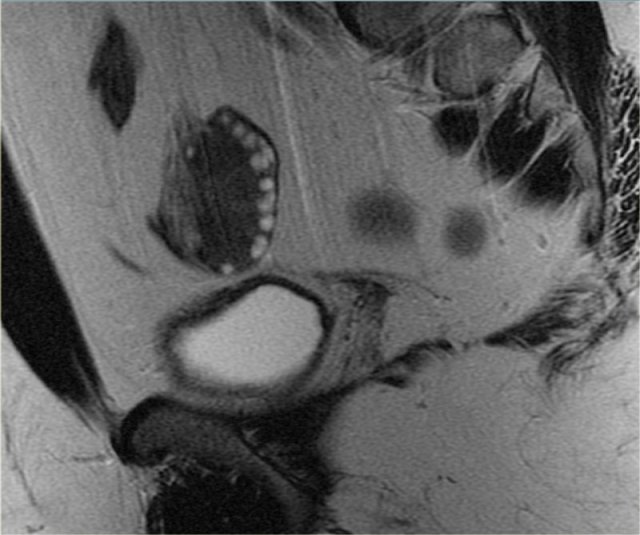
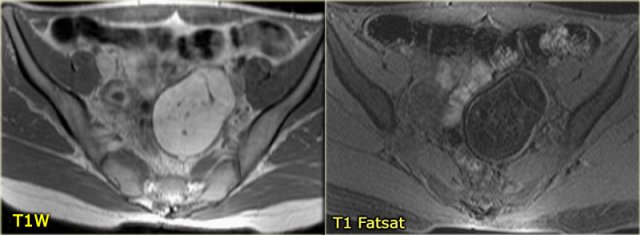
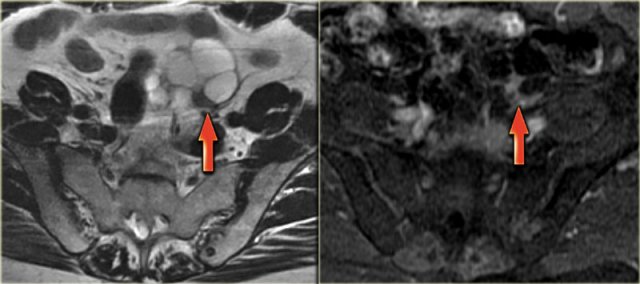
Corpus luteum cyst at MRI: an axial T2-weighted image demonstrating an involuting corpus luteum cyst (arrow).
This is a normal finding.
The right ovary is also normal.
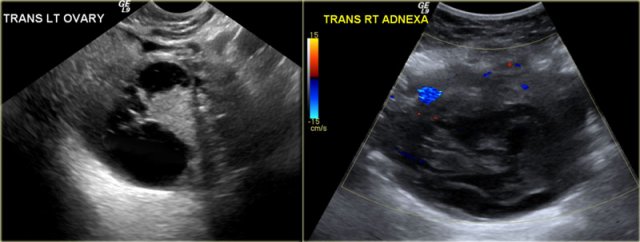
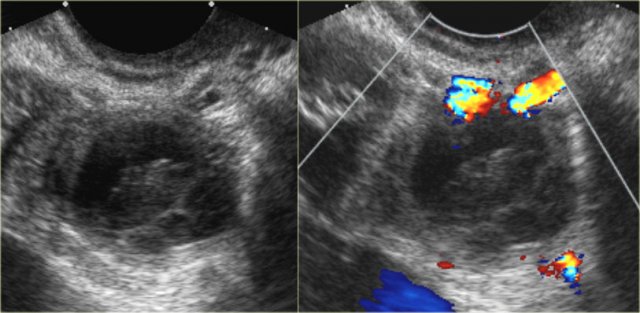 Hemorrhagic cyst with a clot mimicking a neoplasm. Notice absence of flow and good through-transmission
Hemorrhagic cyst with a clot mimicking a neoplasm. Notice absence of flow and good through-transmission
Hemorrhagic ovarian cyst
When a Graafian follicle or follicular cyst bleeds, a complex hemorrhagic ovarian cyst (HOC) is formed.
On ultrasound hemorrhagic ovarian cyst presents as an unilocular thin-walled cyst with fibrin-strands or low-level echoes and good through transmission.
On MRI hemorrhagic cysts are bright on pre-contrast T1-FatSat, and dark on T2.
There should be no internal vascularity on Doppler ultrasound or internal enhancement on CT or MRI.
Hemorrhagic ovarian cysts have variable wall thickness, and often some circumferential vascularity can be seen.
Clinically the classic presentation is with acute pain.
However HOC can also be an incidental finding in an asymptomatic patient.
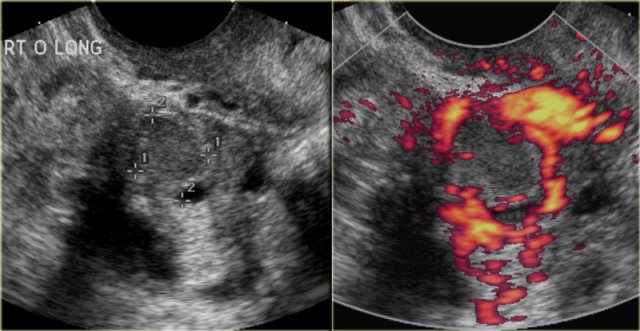
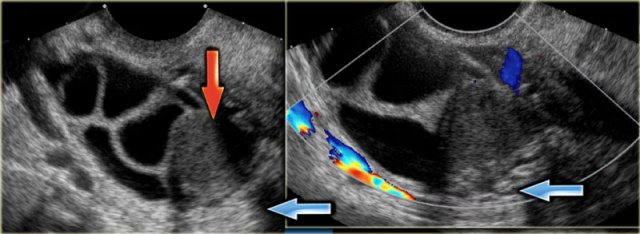
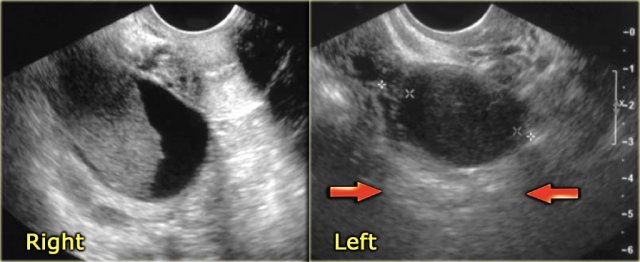
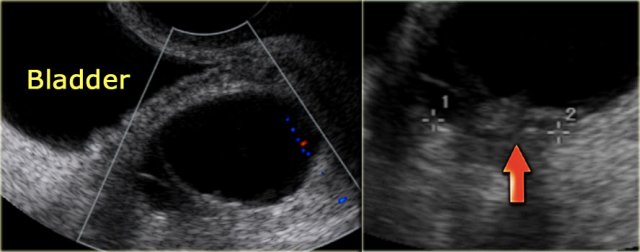
The ultrasound images show multiple simple and one complex right ovarian lesion (red arrow).
The latter demonstrates diffuse low-level echos and no flow on Doppler.
Note that there is a good through transmission (blue arrow).
These findings indicate the presence of a hemorrhagic cyst.
Continue with the MR-images.
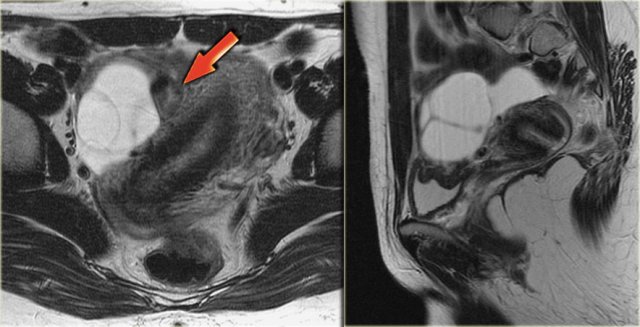
Axial and sagittal T2W images from the same patient.
The right ovary contains multiple simple T2 bright cysts with thin borders and no solid components.
On the axial image there is one lesion, that is dark on T2, i.e. a complex cyst (arrow).
There is a small amount of ascites around the right ovary, but not enough to raise concern of a possible neoplasm.
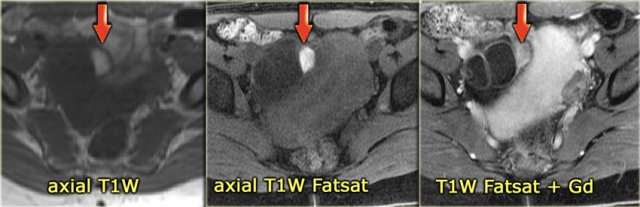
On the T1-weighted image without fatsat the complex cyst is bright, indicating either fat or blood content.
On the T1-weighted image with fatsat the lesion remains bright, ruling out a fatty lesion.
After the administration of Gd there is no enhancement, confirming that this is a hemorrhagic ovarian cyst.
An endometrioma would be in your differential.
Note that subtraction images are best to demonstrate the lack of enhancement in a lesion, that is bright on the pre-contrast T1-weighted image.
The ultrasound images show the right and left ovary: on both sides there is what appears to be a solid lesion.
There is however good through transmission, which indicates that we are probably dealing with hemorrhagic cysts.
On Doppler US (not shown) there was no vascularity.
Continue with the MR examination.
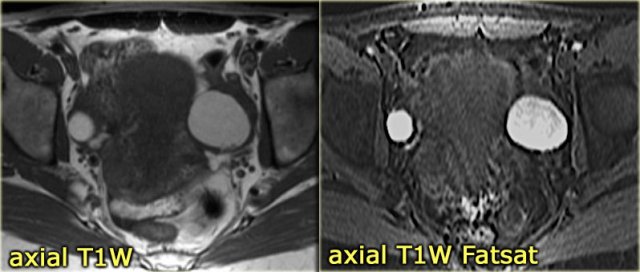
On an axial T1-weighted image both lesions are bright indicating fat, blood or high protein fluid.
Fat saturation does not suppress the signal in these lesions.
In an image with overall reasonably good fat suppression this rules out a fat-containing teratoma and confirms the suggestion of hemorrhagic fluid.
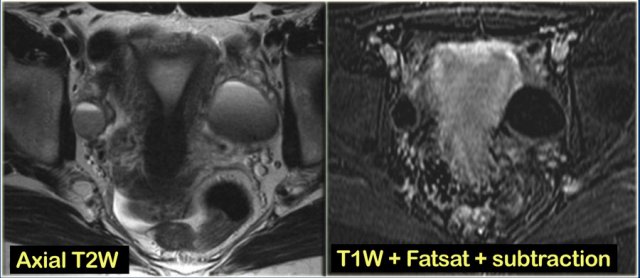
On the axial T2-weighted image both lesions show typical 'shading'.
The gradual drop in T2 is thought to be caused by a combination of increasing viscosity and increasing concentration of protein and iron towards the dependent portion of the lesion.
There is no enhancement on the subtraction image (Post-Gd minus Pre-Gd).
Again, subtraction is useful in cases like this: Gd-induced signal increase over the already very bright pre-contrast image would be very hard to appreciate otherwise.
Other benign cystic and cyst-like lesions
Endometrioma
Cystic endometriosis or endometrioma is a type of cyst formed when endometrial tissue grows in the ovaries.
It affects women during the reproductive years and may cause chronic pelvic pain associated with menstruation.
The ovaries are involved in approximately 75% of patients with endometriosis.
On ultrasound endometrioma can be variable but the great majority (about 95%) of patients present with a classic homogeneous, hypoechoic cyst with diffuse low level echoes.
Rarely it is anechoic, mimicking a functional ovarian cyst.
Endometriomas can be multilocular and have thin or even thick septations.
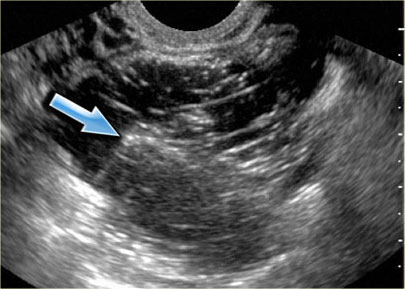
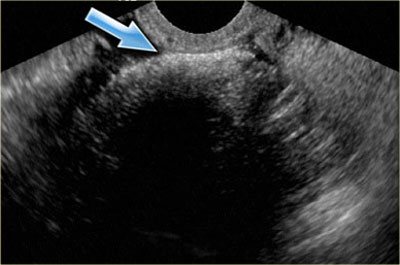
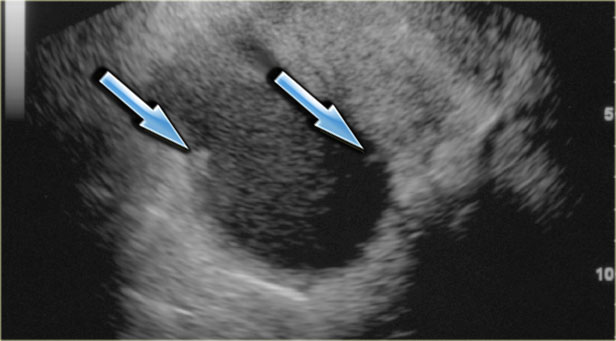 Transvaginal ultrasound: endometrioma with two hyperechoic wall foci, consistent with cholesterol deposits (arrows)
Transvaginal ultrasound: endometrioma with two hyperechoic wall foci, consistent with cholesterol deposits (arrows)
In about one third of patients, on careful examination, small echogenic foci can be seen adhering to the wall.
These have been postulated to be cholesterol deposits, but may also constitute small blood clots or debris.
It is important to differentiate these foci from true wall nodules.
In the presence of these foci, the diagnosis of an endometrioma is very likely.
The transvaginal ultrasound shows a typical endometrioma, with hyperechoic wall foci.
At Doppler US no vascularity was seen in these foci (not shown).
The next case is a transvaginal US-image that shows a cystic lesion with a hyperechoic structure.
There is a wide differential diagnosis including ovarian cystic neoplasm with solid component, mature cystic teratoma with hyperechoic Rokitansky nodule, hemorrhagic cyst with clot and endometrioma with clot or debris.
Continue with the CT and MR.
A CT was requested that turned out to show the same, predominantly cystic lesion.
If additional imaging is needed for cysts that are indeterminate at ultrasound, it is better to perform MRI.
The T2-weighted image on the right correlates nicely with the ultrasound image.
On T2-weighted images endometriomas typically show 'shading'.
MRI confirms the absence of any enhancement, confirming that it is most likely debris within the cyst.
On MRI the hemorrhagic content will make endometrioma appear bright on T1-weighted images.
On T1-fatsat images an endometrioma will remain bright.
This in contrast to teratomas, that are also bright on T1 but dark on T1-fatsat images.
Always include a T1 fat suppressed sequence, because this makes small T1 bright lesions more conspicuous.
The next case is a unilocular, mildly hypoechoic ovarian lesion with through transmission.
There is no internal or wall vascularity on Doppler.
On ultrasound this can again either be a hemorrhagic cyst or an endometrioma.
Continue with the MR images
6 months later a follow-up MRI was performed.
The lesions are bright on T1-weighted images.
The bright signal persists on fat saturation indicating the presence of blood.
There is T2 shading consistent with a hemorrhagic lesion.
There is no enhancement.
The fluid-fluid level in the right ovarian lesion also confirms its cystic nature.
The fact that the lesions persist after 6 months makes bilateral endometrioma much more likely than hemorrhagic cysts.
Polycystic ovary syndrome
The Poly-Cystic Ovary Syndrome (PCOS) is also known as Stein-Leventhal syndrome.
Imaging can confirm or suggest the diagnosis.
Imaging criteria:
- 10 or more peripheral simple cysts
- Usually Characteristic 'string-of-pearls' appearance.
- Ovaries are typically enlarged, although in 30% of patients the ovaries have a normal volume.
These patients usually have menstrual cycle irregularities and either typical clinical signs of hirsutism, obesity, infertility, acne, male balding pattern or biochemically show increased androgen levels.
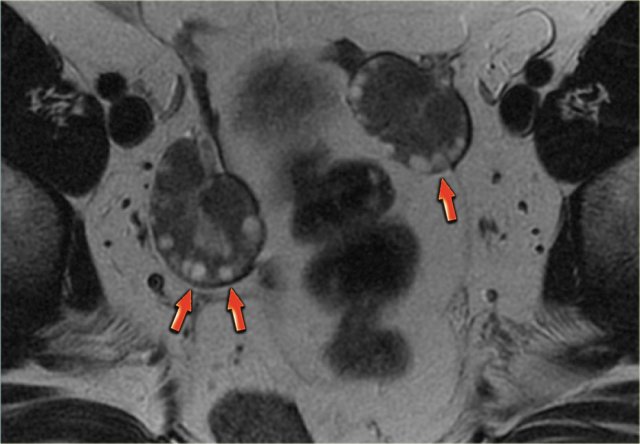
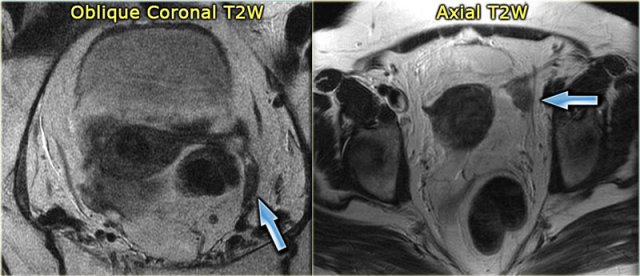
On the left a sagittal T2-weighted image in a patient with increased serum androgen levels.
The ovary is enlarged and shows multiple small peripherally located simple cysts
The obesity associated with this syndrome is evident from the abundance of fat, showing bright on these FSE T2-weighted images.
In this patient, MRI confirmed the diagnosis of PCOS.
Ovarian hyperstimulation syndrome - Theca lutein cysts
Ovarian hyperstimulation syndrome is a relatively rare condition.
It is caused by hormonal overstimulation by hCG, and is therefore usually bilateral.
Hormonal overstimulation can occur in gestational throphoblastic disease, PCOS or in patients receiving hormonal therapy.
It can also be seen in pregnancy, but seldom in a normal single pregnancy. If it does occur in normal pregnancies, the reported natural course is spontaneous resolution after birth.
In normal pregnancies the reported natural course is spontaneous resolution after birth.
Hormonal overstimulation more often occurs in molar pregnancy, erythroblastosis fetalis or in plural pregnancies.
On imaging there is - usually bilateral - ovarian enlargement with multiloculated cyst that can totally replace the ovary.
The clinical history is the distinguishing feature to make the diagnosis of ovarian hyperstimulation syndrome.
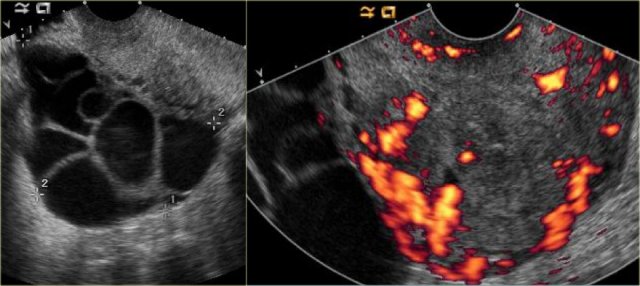 Theca lutein cysts: US images of a young pregnant woman. In both ovaries there are multiple cysts. Right image shows an invasive uterine mass, consistent with invasive molar pregnancy.
Theca lutein cysts: US images of a young pregnant woman. In both ovaries there are multiple cysts. Right image shows an invasive uterine mass, consistent with invasive molar pregnancy.
The US images are of a young pregnant woman, who had multiple ovarian cysts. The other ovary is not shown but showed a similar appearance.
The features needed to make the diagnosis of ovarian hyperstimulation syndrome are in the clinical history - a young pregnant woman - and in the last image of the uterus, which shows an invasive uterine mass, consistent with invasive molar pregnancy.
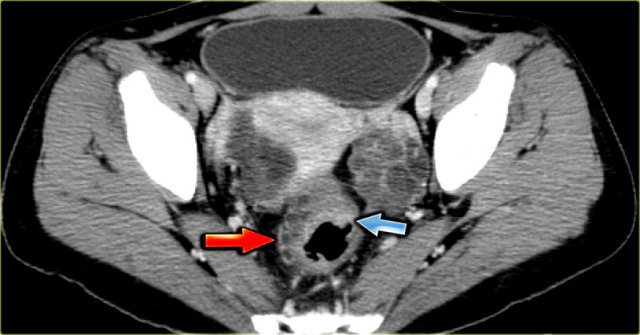
PID with tubo-ovarian abscess
Tubo-ovarian abscess (TOA) usually arises as a complication of Chlamydia or Gonorrhoeae infection that rises from the vagina or cervix to the fallopian tubes.
On imaging a thick-walled complex cystic ovarian lesion is seen with abundant flow.
The presence of a thickened endometrium or hydrosalphinx makes the diagnosis of a PID more likely.
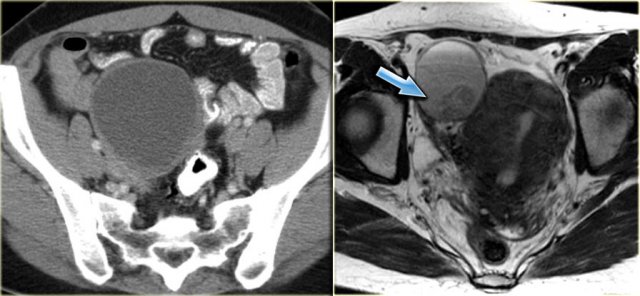
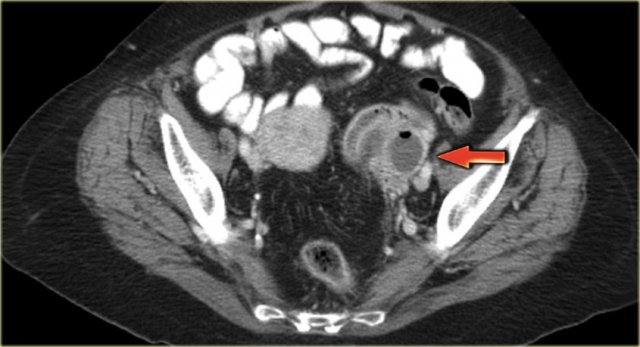
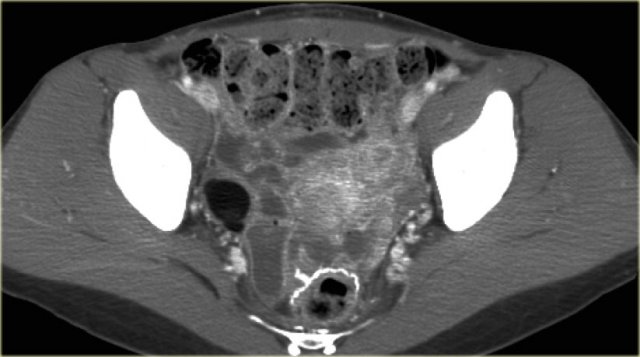
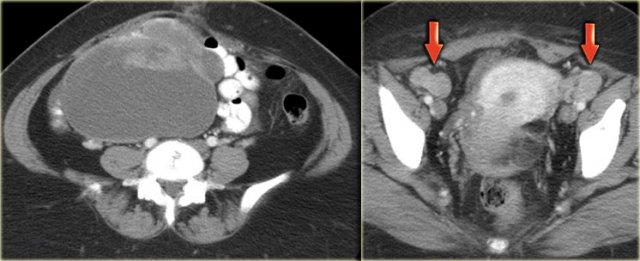
The axial CECT image shows a left complex cystic lesion with thick enhancing walls and internal gas.
It looks like an abscess.
Note the relatively unremarkable aspect of the overlying mesentery: this is unlikely to be a peri-diverticular abscess.
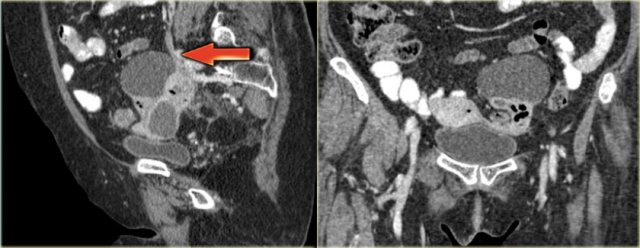
Continue with the reconstructed images.
On the sagittal image notice, that the lesion is connected to the ovarian vein confirming that this is an ovarian lesion (arrow).
The coronal image shows the anatomic connection to the uterus.
There is a gasbubble in the uterine cavity, which confirms the suggestion of an infection rising from the uterine cavity via the salphinx to involve the ovary (click or tap the image to enlarge).
Benign cystic ovarian neoplasms
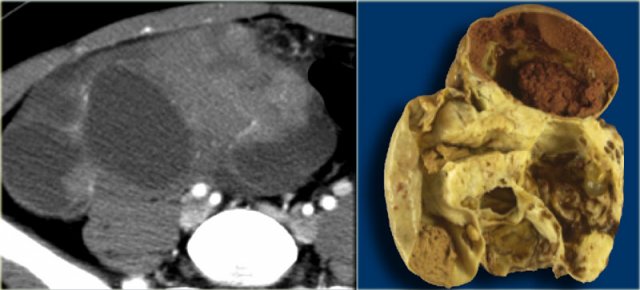
Mature cystic teratoma
A very common benign ovarian lesion that may appear cystic is a mature cystic teratoma, also called dermoid cyst.
Mature in this context means benign, as opposed to the immature, malignant teratoma.
Benign cystic teratomas typically occur in young women of child-bearing age.
At imaging they are usually unilocular (up to 90%) but can be multilocular, and are bilateral in ~15%.
Up to 60% may contain calcifications.
The cystic component is fluid fat, produced by sebaceous glands in cyst lining.
The presence of fat is diagnostic.
The characteristic ultrasound appearance is that of a cystic mass, with a hyperechoic solid mural nodule, which is called a Rokitansky nodule or dermoid plug (figure).
In another case the transvaginal ultrasound shows the 'tip-of-the-iceberg' sign: acoustic shadowing from the hyperechoic part of the dermoid cyst.
This may be misinterpreted as bowel gas and the lesion may be overlooked.
A fat-fluid level may be present, caused by fat floating on more aqueous fluid.
Multiple thin, echogenic lines or stripes may be seen, caused by hair floating in the cyst cavity.
Mature cystic teratomas, even though benign, are often resected because of increased risk of ovarian torsion, the most commonly associated complication.
Other complications associated with teratoma are infection, rupture (spontaneous or trauma) and, rarely, hemolytic anemia (resolves with resection).
Malignant transformation can occur but is also rare (
Axial T1-weighted image in the same patient shows a bright lesion with an internal septation.
A septation is seen in about 10% of thse lesions.
On the T1-weighted image with fat suppression there is suppression of the signal.
This confirms the fatty content and is diagnostic of a teratoma.
Classic low attenuation consistent with fat in a right sided cystic teratoma at CT.
Cystadenoma and cystadenofibroma
Cystadenoma and cystadenofibroma are also common benign ovarian tumors. They can be either serous or mucinous.
At imaging a serous cystadenoma is most often unilocular and anechoic, and may look like a simple cyst.
Mucinous cystadenomas are most often multilocular with thin (
The locules may contain complex fluid, due to proteinaceous debris or hemorrhage, or both.
The finding of papillary projections should raise the suspicion of a possible borderline malignancy or a cystadenocarcinoma.
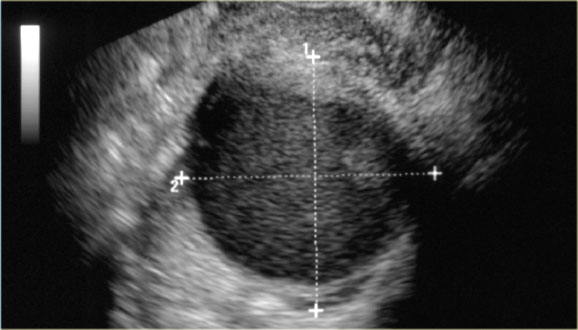
Transvaginal ultrasound shows a 5.1x5.2-cm dominant left ovarian cyst.
The cyst is anechoic and no septations are seen.
Also there is no ascites.
There is, however, a nodule on the posterior wall that shows no flow on Doppler.
This may be a follicular cyst with some debris, but a cystic neoplasm cannot be excluded.
Work-up with MRI is recommended.
T2-weighted image of the same patient shows thin enhancing septations (as well as motion artifacts that should not be mistaken for septations).
There are no tumor nodules and no adenopathy or peritoneal deposits.
There is only a small amount of ascites.
This proved to be a cystadenoma.
The next case is another cystic lesion
On the posterior wall a solid mural nodule is found, which is avascular.
No secondary signs of malignancy.
Continue with the MRI.
Five years later the lesion has grown.
Axial T2 shows a complex cystic left ovarian lesion, with a solid nodule on the posterior wall.
At post-contrast axial T1W-FatSat the thin septa and the mural nodule show slight enhancement.
On the basis of these findings the distinction between a benign ovarian lesion such as a cystadenofibroma and a malignant lesion cannot be made.
The lesion was resected and was found to be a cystadenofibroma.
The next case is a transabdominal ultrasound that shows a left-sided multiloculated cystic mass.
This looks like a cystic ovarian neoplasm but no ovary could be identified.
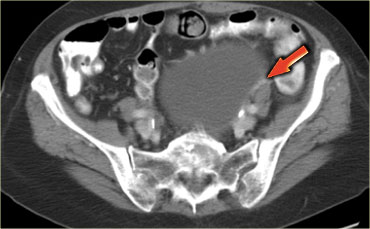
CT of the same patient shows a multi-loculated cystic mass adjacent to the bladder, connected to the left ovarian vein (arrow).
There are thick septations and irregular wall thickening.
On the basis of this CT the distinction between a benign ovarian lesion such as as cystadenofibroma and a malignant ovarian lesion cannot be made.
The lesion was resected and found to be a cystadenofibroma.
Malignant cystic ovarian neoplasms
Remember, the role of imaging is not to determine the histological nature of a lesion, but to distinguish benign from malignant lesions and guide decisions on further management.
The examples given here serve as a demonstration of suspicious imaging features, not as a guide for determination of histologic lesion type.
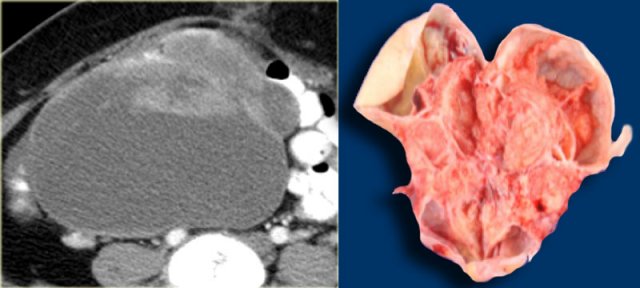
Serous ovarian cystadenocarcinoma
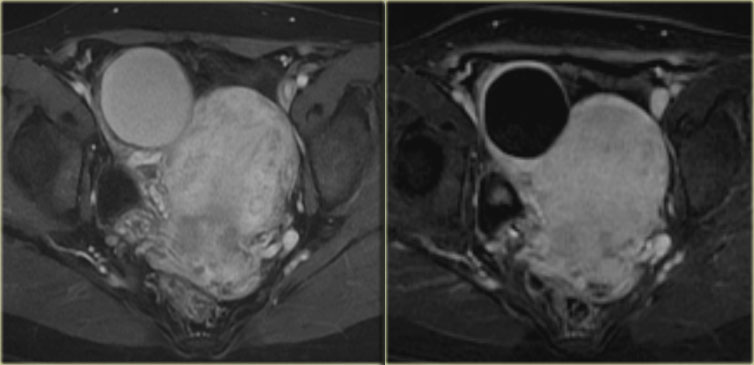
Ultrasound shows a complex solid-cystic mass in the left ovary, and another, very large complex solid-cystic mass in the right hemi-pelvis.
CT of the same patient shows a complex solid-cystic mass with thick, enhancing septations in the right ovary.
These findings are very suspicious for a malignant cystic neoplams.
There is also bilateral lymphadenopathy (arrows).
Pathology showed a serous ovarian cystadenocarcinoma.
This is the most common type of ovarian cancer.
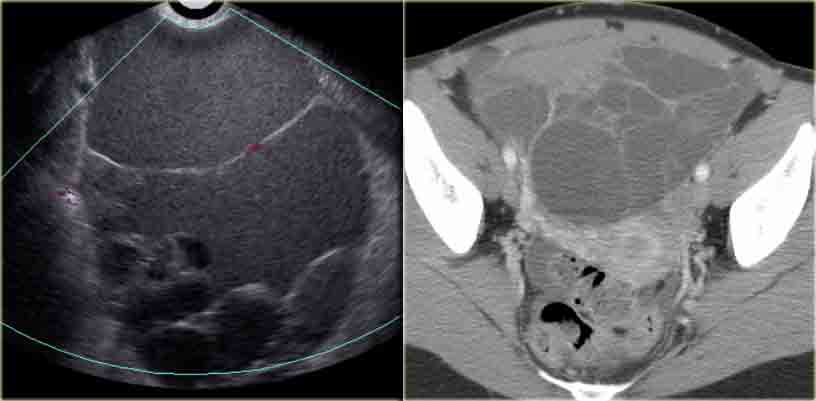
Mucinous ovarian cystadenocarcinoma
Ultrasound shows a very large multi-loculated cystic lesion in the region of the right adnex.
Some locules are anechoic. Others contain uniform low-level echoes, consistent with proteineous content, such as hemorrhage or, in this case, mucin. The septations are thin, except for the dorsal septations that appear somewhat thicker, partially caused by the lower scanresolution at great depth.
The septations are avascular.
There are no solid components.
There was no ascites.
Despite the absence of solid components and despite the absence of vascularity on color Doppler, the size and the multi-loculated aspect of this lesion are suspicious for a cystic neoplasm and warant further evaluation.
The CECT shows similar findings.
The locules are of different attenuation, consistent with varying protein content.
There is no ascites orperitoneal deposits and no lymphadenopathy.
At pathology this was a mucinous cystadenocarcinoma of low malignant potential.
The thin, relatively avascular septae, the absence of frank solid components, the absence of ascites and peritoneal carcinomatosis and the absence of invasion, suggest a lesion of low malignant potential (LMP).
Note however, that this diagnosis can not be made on imaging findings alone.
Especially the absence of invasion in ovarian stroma cannot be judged reliably on imaging.
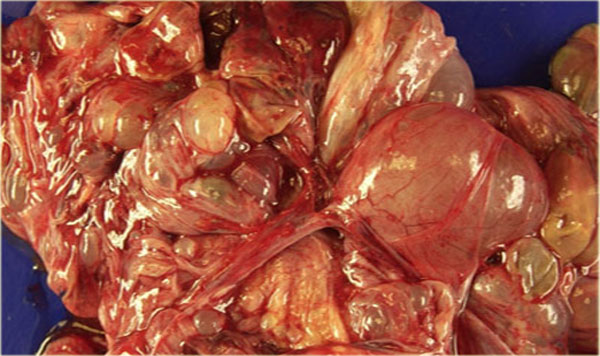
Endometrioid ovarian carcinoma
On ultrasound both ovaries are markedly enlarged and contain cystic components with intracystic solid components (arrows).
The complex solid-cystic lesions, in addition to being bilateral, are suspicious for a cystic ovarian neoplasm and warrant further evaluation.
Again, the role of imaging is to confirm a lesion is present and to decide that this is not a lesion that can be classified as definitely benign nor a lesion that can be safely followed-up: action is required.
CT of the same patient confirms large bilateral complex solid-cystic lesions, bulging into the abdomen.
The purpose of the CT is not to confirm what was already known from the ultrasound, but to stage disease.
On the basis of CT (or of MRI) it is not possible to determine the histologic type of the tumor.
This is not relevant. This patient will undergo surgery.
For epithelial tumors - by far the most common group of malignant ovarian tumors - even after surgery, the exact tumor subtype is much less important for the prognosis than factors such as FIGO-stage, tumor differentation grade, and how succesful surgery was in removing all of the disease.
For this patient the relevant findings are on the image on the left.
There is a peritoneal implant.
The tumor was resected and pathology showed this was an endometrioid ovarian carcinoma.
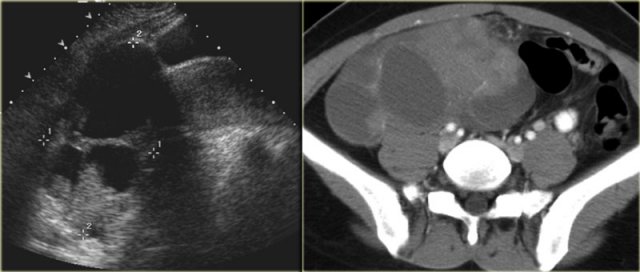
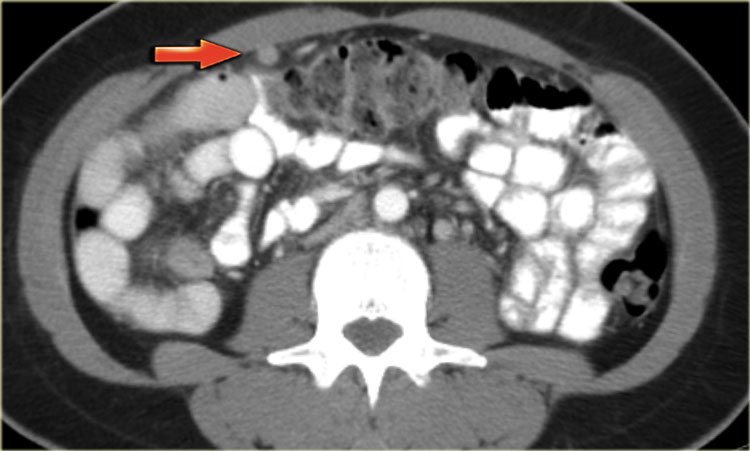
Cystic metastases to the ovaries
While metastases to the ovary are most commonly solid - such as for example Krukenbergs metastases - cystic ovarian metastases do occur.
The CT image shows complex cystic masses in both ovaries.
While a serous cystadenocarcinoma may very well be bilateral, they are more often unilocular than multilocular.
Barely visible is part of a circumferential colorectal cancer (blue arrow).
Clearly visible are cystic implants on the peritoneal reflection (red arrow).
These were cystic ovarian metastases of a colorectal cancer.
This is an uncommon finding.